Notes for Macromolecules are available for class 12 Students. it is chapter 14 (FSc). Visit the Umair Khan academy website or Youtube Channel for more notes and FSc preparation.
The following are the most important short questions to best prepare for exams with Video discussion for your easy understanding.
Q1: What are macromolecules?
Molecules having molecular masses usually more than 10,000 are called macromolecules. Carbohydrates, proteins, lipids, steroids and hormones etc.
Q2: Define Polymerization.
Any process in which monomers combine chemically to produce a polymer is called polymerization. The monomer molecules present in the polymer usually number from at least 100 to too many thousands.
Q3: What is the degree of Polymerization?
The number of repeating units in the chain which determines the length of the polymer chain is called the degree of polymerization. Abbreviated as D.P
Q4: How the Degree of polymerization help to determine the molar mass of the polymer?
The sum of all the atomic masses present in the molecules of a polymer is the molar mass of the polymer.
The molar mass of polymer = molar mass of monomer x Degree of polymerization
Q5: How the polymers are classified on the bases of heat effects?
Thermoplastic polymers soften on heating and become rigid again on cooling like PVC nylon etc Thermosetting polymers become hard on heating and softness cannot be gained. polyurethanes or epoxy resins are the best examples.
Q.6: What is the difference between homopolymers and copolymers
In homopolymers, the same types of monomers are present while in copolymers two types of monomers are present polyethene is a homopolymer and polyester is a copolymer.
Q.7: What is condensation polymerization?
In condensation polymerization, different types of monomers are used and the involvement of small molecules like H₂O, CH3-OH and HCI etc.
Q.8: What are polyamides? Give the formation of nylon 6,6.
In polyamides, the carbonyl group is attached to the amino group and two different types of monomers are involved. Reaction as follows.
Q9: How PVC is prepared? Give its uses.
Vinyl chloride is subjected to high pressure and high temperature to produce polyvinyl chloride.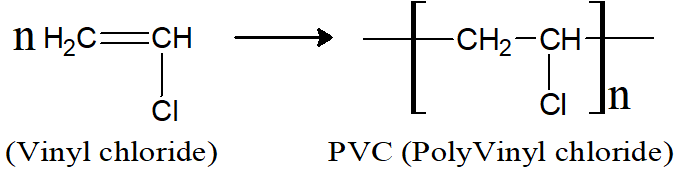
PVC is used for making gramophone records, floor coverings and rubber-like textures.
Q10: How polystyrene is prepared? Give its uses.
It is a polymer of styrene. It is prepared by heating styrene at 132°C. Polystyrene is used in the packing of electrical equipment, insulation of heat, manufacture of food containers and preparation of cosmetic bottles. It also used to prepare television cabinets and plastic cups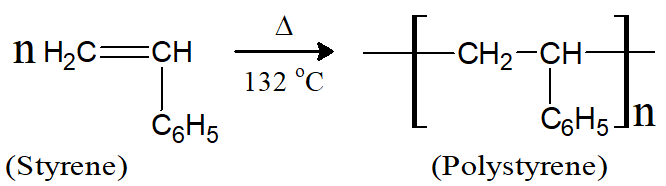
Q11: What is meant by plant starch and animal starch?
The mixture of amylase and amylopectin is called plant starch. The glycogen which is stored in animals is called animal starch.
Q12: Which monomers are formed by the hydrolysis of lactose and sucrose?
- Lactose on hydrolysis changed to glucose and galactose.
Lactose + H2O → Glucose + Galactose - Sucrose on hydrolysis changes to fructose and glucose.
Sucrose + H2O → Glucose + Fructose
Q.13: What is the difference between cellulose and starch?
Both cellulose and starch are polysaccharides. Both on hydrolysis give glucose. In cellulose, monomers are β-D-glucose while in starch the monomer is α-D-glucose.
Q.14: What is the difference between α -D-glucose and β -D-glucose?
Both are cyclic forms of glucose. In both molecules position of the -OH group is different at carbon 1.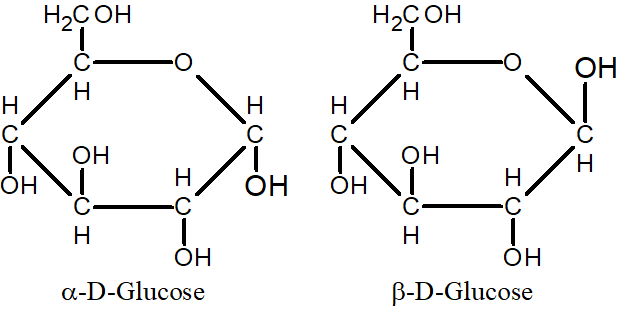
Q15: What is the difference between simple proteins and compound proteins?
The proteins which give only amino acids on hydrolysis are called simple proteins, e.g. albumin, and globulin.
The protein which on hydrolysis give amino acids and some other non-protein portions are called compound proteins, e.g., phosphoprotein, lipoproteins
Q.16: What are epoxy resins? Give their uses
Epoxy resins are polymers. They are called so because the starting material in them is epoxide. They are prepared by the condensation polymerization of chloro-epoxy alkane with dihydric phenol.
Q.17: What are aldoses and ketoses? Give example.
Those monosaccharides which have an aldehyde group in them are called aldoses like glucose. And those monosaccharides which have a ketonic group in them are called ketoses like fructose.
Q.18: What is the basic skeleton present in steroids?
The parent nucleus of steroids consists of three six-membered rings and one five-membered ring. This cycle is named perhydrocyclopentanophenanthrene.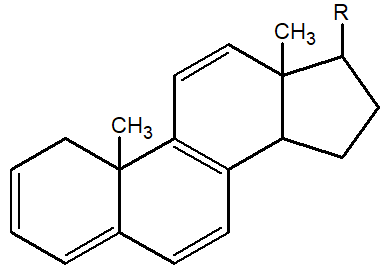
Q.19: What do you mean by the denaturation of proteins?
It is the structure disruption of proteins by heat, by changing pH or by using strong oxidizing and reducing agents. Albumin is a protein; when egg Albumin is heated, it hardens. this change is denaturation and irreversible.
Q.20: What do you mean by hydrolysis of fats and oil?
Fats and oils are hydrolyzed by enzymes which act as catalysts. These enzymes are called lipases. This hydrolysis takes place in the digestive tract of human beings & animals:
Q.21: What is saponification?
It is the hydrolysis of triglycerides by alkalies to produce glycerol and soap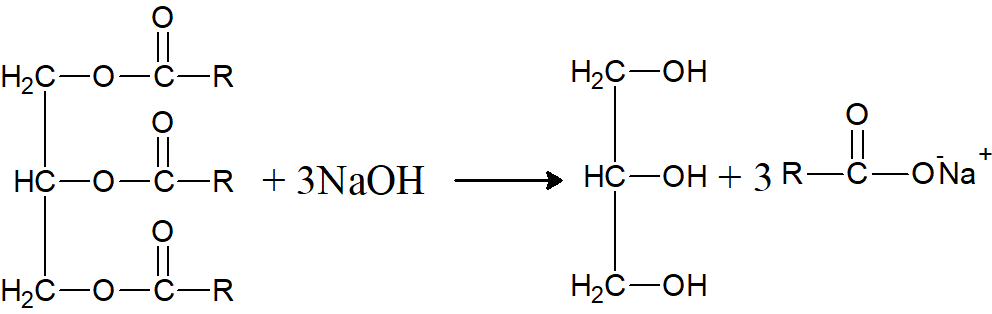
Q.22: What do you mean by hardening of oils?
The conversion of unsaturated glycerides by passing Hydrogen, in the presence of a nickel catalyst into saturated fats is called the hardening of oil.
Q.23: What is the Saponification Number?
The number of milligrams of KOH is required to saponify one gram of fat & oil. The general formula is
Saponification Number = 168000/ molar mass of fat
Q.24: What do you mean by the rancidity of fats and oils?
The process of spoilage of fats and oils with an odour is called rancidity. It is due to the hydrolytic reaction due to oxidation. In this reaction, a foul smell of aldehyde and fatty acids is produced.
Q.25: What are Steroids? Give example.
Steroids are lipids, and they are naturally occurring. Their nucleus consists of four rings and these rings are fused with each other. Important steroids are Cholesterol, Ergosterol, and male and female sex hormones.
Q.26: What is the acid number of fats?
It is the number of mg of KOH which is required to neutralize one gram of fat. It gives us information about the number of fatty acids present in it. It also tells us about the extent of rancidity.
Q.27: What do you mean by iodine number?
It is the number of grams of iodine which will react with 100 grams of fats and oils. Iodine saturates the double bond and from the amount of iodine absorbed, we can calculate the amount of unsaturation.
Q.28: What is the chemical nature of enzymes?
Enzymes are either pure protein or contain protein as well as some non-protein portion essential for the activity of enzymes. The protein component of an enzyme is called an apoenzyme and the non-protein portion of the enzyme is called a co-factor or co-enzyme.
Q.29: What are the optimum conditions of temperature and pH for the activity of enzymes?
Enzyme reactions occur best at 37°C which is the normal human body temperature. Enzymes usually destroy at high temperatures. The activity of the enzyme is maximum in neutral, slightly acidic or slightly basic medium. In highly acidic or highly basic mediums their activity decreases. For example, the optimum pH for salivary amylase is 6.4 to 6.9.
Q.30: What is the effect of radiation on the enzymes?
Generally, enzymes are readily inactivated by exposure to ultraviolet light. Beta rays, gamma rays and X-rays.
Q.31: Give types of Nucleotides.
There are two types of Nucleotides in DNA and RNA. The only difference between Deoxyribose nucleic acid and ribose nucleic acid is a difference in Sugar They are found as a part of the conjugated proteins and are called nucleoproteins. Nucleic acid directs the synthesis of proteins.
Q.32: What is the difference between a glycosidic linkage and a peptide linkage?
Glycosidic linkage: This is present in oligosaccharides and polysaccharides. Whenever monosaccharides combine together with the elimination of the H₂O molecule, the resulting bond is Glycosidic. as in the sucrose molecule.
Peptide linkage: This linkage is present in proteins. Amino acids combine through a peptide bond with the elimination of water molecules e.g. dipeptides.
Q.33: What are Nucleotides?
A nucleotide is a combination of a phosphate group and a Nitrogenous base with sugar. Sugar may be ribose or deoxyribose. so, they can be called ribonucleic acid (RNA) or Deoxyribonucleic acid (DNA)
Q.34: What is the difference b/w DNA and RNA?
DNA carries the genetic information while RNA is involved in putting this information together. The sugar part of two nucleic acids in RNA is ribose, while the sugar in DNA is deoxyribose. DNA is always double-stranded while RNA is single-stranded. DNA is only present in the nucleus while RNA is present throughout the cell.