Halogens and noble gases is chapter 5 in class 12 chemistry. For more lectures and notes from Umair Khan Academy, visit UmairKhanAcademy or its Youtube Channel.
The following are the most important short questions for exam preparation from Halogens and Noble Gases with Video discussion below.
Q.1: What is bleaching powder?
Bleaching Powder
The chemical name of the bleaching powder is calcium hypochlorite chloride [Ca(OCl)Cl] it is a yellowish-white powder with a strong smell of chlorine.
Q.2: How bleaching powder is prepared commercially?
Commercial Preparation
Bleaching powder can be prepared by the reaction of chlorine with dry-slaked lime using one of the following methods
- Hasenclever’s method
- Beckmann’s method
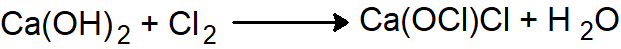
Q.3: Give uses of bleaching powder
Bleaching powder is used:
- For the laboratory preparation of the chlorine and oxygen.
- It is also used in the manufacturing of chloroform.
- As a disinfectant and in the sterilization of water
- For making unshrinkable wool
- For bleaching cotton linen and paper pulp.
Q.4: Write a few lines about the oxides of chlorine.
The oxides of chlorine are generally unstable. It is not possible to synthesize them by direct combination of the elements of chlorine and oxygen. They have extensive industrial use as commercial bleaching agents for wood, paper- pulp and for water treatment.
Q.5: What is disproportionation reaction explain your answer with an example.
The type of reaction in which a specie (molecule atom or ion) is simultaneously oxidized and reduced is called a disproportionation reaction.![]()
The above reaction is a disproportionation reaction because the zero Oxidation state of the chlorine atom in Cl2 is converted to -1 in the Chloride and +5 in chlorate
Q.6: Write oxyacids and salts of halogens with the oxidation states of halogen in them.
| Oxidation state | +1 | +3 | +5 | +7 |
| Acids | Hypohalous Acid | Halous Acid | Halic Acid | Perhalic acid |
| Salts | Hypo__ite | ____ite | ___ate | Per___ate |
| e.g | HClO HBrO | HClO2 | HClO3 HBrO3 | HClO4 HIO4, H5IO6 |
Q.7: How are the halogen acids ionized in water?
Hydrogen halides in the water give hydrofluoric acid, hydrochloric acid, hydrobromic acid, and hydroiodic acid. Hydrofluoric acid is a weak acid due to the Limited ionization. The other three acids are very strong. The strength of the acids increases from HF to HI.
HF < HCl < HBr < HI
HF is a weak acid and hydrogen iodide is the strongest acid.
Q.8: Why HF is a weaker acid than HCl?
The tendency of HF to give H+ in water is comparatively low than HCl due to strong hydrogen bonding between the molecules. Therefore, HF produces a low concentration of hydrogen ions in the water as compared to HCl so it is a weaker acid than HCl.
Q.9: Write down the commercial uses of halogens.
- Fluorine is used to prepare Freon, Teflon Halothane and fluorides.
- Chlorine is used in manufacturing bleaching powder, polyvinyl chloride, chloroform and carbon tetrachloride.
- Bromine is used to prepare ethylene dibromide, Silver Bromide and fungicide.
- Iodine is used in the pharmaceutical industry and it is used to disinfect the surface and used as a germicide and tincture of Iodine.
Q.10: What are noble gases? Explain their inertness based on their electronic configuration.
In the periodic table, the elements of group VIII-A are called noble gases or rare gases. They have a very stable electronic configuration as their valence shell are filled therefore they do not react under ordinary conditions.
Q.11: What is “iodized salt”?
When common salt (NaCl) contains 0.02% KI by weight it is called iodized salt. It is observed that when there is no iodide ion in the diet it leads to the enlargement of the thyroid, a condition called goiter.
Q.12: What is Freon and Teflon?
Freon
Freon is the commercial name of the low molecular mass fluoro-chlorocarbons. Chlorine is used to prepare Freon.
Examples are CF2Cl2 (Dichlorodifluoromethane), and CF3Cl (Chlorotrifluoromethane).
Freon is being used as a refrigerant and aerosol propellant.
Teflon
Tetrafluoroethylene is polymerized to form Teflon. It is a valuable plastic which resists the action of oxidants, acids and alkalis.
Q.13: Arrange the halogen ions in order of their increasing size.
F– < Cl– < Br – < l–
Q.14: Why iodine has metallic lustre?
Atoms of iodine are larger in size and their valence electrons are not tightly held with the nucleus. When these electrons absorb energy, they get excited and when they de-exited emit the energy in the form of visible radiation. These radiations give Grayish black metallic luster to Iodine.
Q.15: Which halogen sublimes to violet vapours?
Iodine is solid when heated, it directly converts into vapours and its colour appears violet.
Q.16: Which halogen is used as an antiseptic?
Both chlorine and bromine are used as antiseptics.
Q.17: Which halogen is used in water treatment to kill bacteria?
Chlorine is used in water treatment to kill bacteria.
Q.18: Name the gas which is used for earthquake prediction.
Radon being radioactive is used for earthquake prediction
Q.19: Name the gas which is used in bacterial lamps.
Xenon is used in bacteria lamps
Q.20: Arrange the oxyacids in increasing order of acid strength and oxidizing power.
HClO4 > HClO2 > HClO
Q.21: Write the reaction of sodium hydroxide with chlorine in a cold State.
![]()
In a cold state at 15°C chlorine will react with Sodium Hydroxide to form hypochlorite and sodium chloride. The reaction is a disproportionation reaction because the zero Oxidation state of the chlorine atom in Cl2 is converted to -1 in Chloride and +1 in hypochlorite.
Q.22: Write down any four applications of noble gases.
- Helium is used in weather Balloons welding and traffic signal lights.
- Helium is used as a cooling medium for nuclear reactors.
- Neon and Helium Arc is used in making glass laser.
- Argon is used for welding and cutting.
- Xenon is used in bacteria lamps.
Q.23: Give one method of preparation and one use of I2O5.
It can be prepared by heating iodic acid at 240°C.![]()
It is used for the quantitative analysis of CO.![]()
Q.24: How bleaching powder reacts with Ammonia and excess amount of sulphuric acid.
Bleaching powder oxidises Ammonia to nitrogen.![]()
If the excess sulphuric acid is added to the bleaching powder it produces chlorine.![]()
Available chlorine
The amount of chlorine thus set free is called available chlorine. The activity of bleaching powder is measured in terms of available chlorine the average percentage of available chlorine in Bleaching powder is 35 to 40%
Q.25: HXO4 is strongest oxyacid. Explain?
The acidic strength increases with the increase in the number of oxygen atoms, as the oxidation state of the halogen increases the bonding electron is shifted away from the H-atom and the tendency of the molecule to lose Proton increases this account for the change of the strength of oxyacids HXO4 has four oxygen atoms so it is the strongest oxyacid.
Q.26: What is the Peculiar behaviour of fluorine?
Fluorine differs from the other halogen in many respects which is due to,
- The small size of a fluorine atom and fluoride ion.
- High first ionization energy and electronegativity.
- The low dissociation energy of fluorine molecule as compared to the chlorine and bromine.
- For restriction of the valence shell to an octet.
- Direct combination with inert gases.
Q.27: Why fluorine acts as a strong oxidizing agent?
The oxidizing power of fluorine is higher because it has low energy of dissociation and higher hydration energy of its ions. Due to the relative strength of oxidizing agents, it is possible for each free halogen to oxidize the ions of the Other halogen next to it in the family. Fluorine can oxidize all the halide ions to molecular halogens.
Q.28: Write four properties of Hydrogen fluoride.
Hydrogen fluoride has the following properties,
- It is a colourless volatile liquid
- It attacks glass and has found application as a non-aqueous solvent.
- It has a melting point of -83.8 °C
- It has a boiling point of 19.5 °C
Q.29: What is halothane, and what is the formula? Give its uses.
Chemical compounds of halogens with ethylene are called halothane. Its example is Teflon (-CF2-CF2-)n. Corrosion-proof parts of the machinery are made of it. Teflon is used in non-stick coating for cooking pans.
Q.30: What are the major applications of neon?
Neon is largely used in making advertising signs in high-voltage indicators and TV tubes.
Neon and Helium are used in making glass lasers.
Q.31: Write two uses of Krypton.
Krypton is used to fill fluorescent tubes and flash lamps for high-speed photography.
Q.32: Write formulas of two oxides of bromine.
- Bromine monoxide (Br2O)
- Bromine dioxide (BrO2)
- Bromine trioxide (Br3O8) or BrO3
Q.33: Why the lattice energy of fluorides is greater than chlorides?
Due to the small size of fluoride Ion, there will be a better overlap of orbitals and consequently leading to a shorter and stronger bond with other elements. Ionic fluorides have higher lattice energy than chlorides and the value is responsible for the insolubility of the fluorides in water. Due to the low dissociation energy of the fluorine molecule, it is highly reactive. The other halogens react slowly under similar conditions. The fluorides are, however more stable with respect to the dissociation into elements.