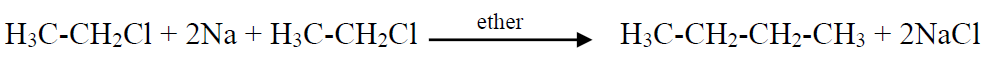Umair Khan Academy is providing ‘Alkyl halides’ notes, for its valuable students. Alkyl halide is an essential chapter from the examination point of view. For more notes on different chapters visit here.
The Short question will be available shortly…
INTRODUCTION
Q1. Define Haloalkanes
Derivatives of hydrocarbons in which hydrogen atoms are substituted by halogen atoms.
Q2. Write down the Classification of Haloalkanes (Based on number of halogens)
Based on the number of halogen atoms present in the molecule, haloalkanes can be classified as;
- 1. Mono-halo-alkanes or alkyl halides
- 2. Di-halo-alkanes
- 3. Tri-halo-alkanes
- 4. Poly halo-alkanes
Q3. Write a few lines on alkyl Halides or Mono-halo-alkanes
It Contains only one halogen atom.
General Formula
The general formula is R-X where -R may be methyl, ethyl, propyl etc. while X represents a halogen atom (F, Cl, Br, I).
Q4. Write the Classification of Alkyl Halides (Based on the nature of carbon atom)
The nature of the carbon atom is different to which the halogen atom is attached.
1- Primary Alkyl Halides:
The alkyl halide when a halogen atom is attached to carbon which is further attached to one carbon or no carbon atom is called primary alkyl halide.
Example: CH3—Cl (Chloromethane)
2- Secondary Alkyl Halides:
The alkyl halide when a halogen atom is attached to carbon which is further attached to two carbon
atoms is called secondary alkyl halide.
Example: CH3CHClCH3 (2-Chloropropane)
3- Tertiary Alkyl Halides:
The alkyl halide when a halogen atom is attached to the carbon which is further attached to three carbon
atoms is called tertiary alkyl halide.
Example: (CH3)3CCl (2-Chloro-2-methylpropane)
NOMENCLATURE OF ALKYL HALIDES
Q5. Write a few Common Names of alkyl halide.
Named according to the nature of the alkyl group to which the halogen atom is attached.
Example:
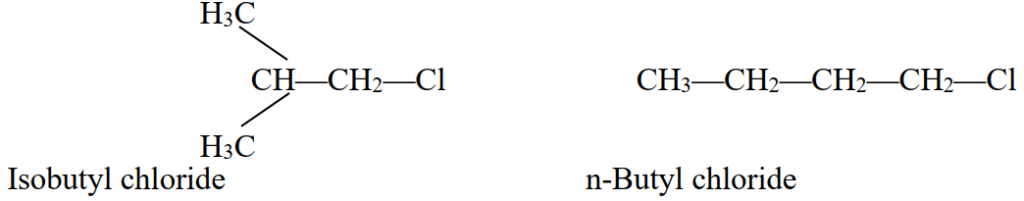
Q6. Write the IUPAC nomenclature of alkyl halide.
- Select the longest continuous carbon chain.
- Gives the lowest number to carbon which is directly attached to the halogen atom.
- If the same alkyl substituent occurs then use prefix di, tri, etc.
- The position of the substituent is indicated by appropriate numbers separated by commas. If the same substituent occurs twice on the same carbon atom, the number is repeated.
Example:
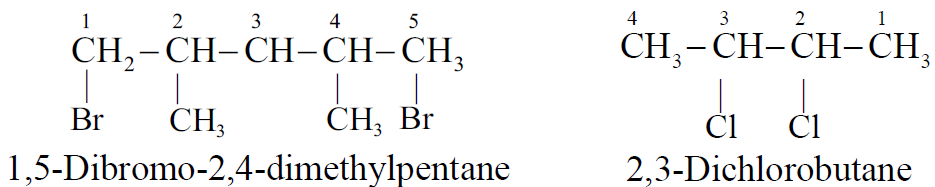
METHODS OF PREPARATION OF ALKYL HALIDES
Q7. Write the IUPAC nomenclature of alkyl halide.
From Alcohols:
a) Reaction of Alcohols with Halogen acids
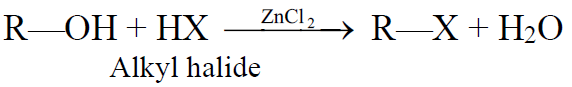
b) Reaction of Alcohols with Thionyl chloride
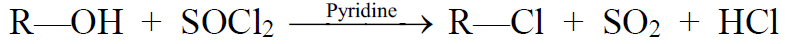
Reaction of Alcohols with Phosphorus Halides:
c) Phosphorus trihalide:

d) Phosphorus pentahalides:
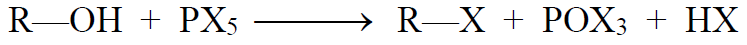
From Alcohols:

REACTIVITY OF ALKYL HALIDES
-
Factors affecting the reactivity:
- C—X Bond Energy:
R—I > R—Br > R—Cl > R—F - C—X Bond Polarity:
R—F > R—Cl > R—Br > R—I
- C—X Bond Energy:
- Experimental conclusion:
Experiments have shown that the strength of carbon halogen bonds is the main factor
which decides the reactivity of alkyl halides. - Order of Reactivity:
The overall order of reactivity of alkyl halides for a particular alkyl group is:
R—I > R—Br > R—Cl > R—F
Q8. Define each with one example. 1. Primary alkyl halide 2. Secondary alkyl halide
1- Primary Alkyl Halides:
In primary alkyl halides, the halogen atom is attached with a carbon which is
further attached to one carbon or no carbon atom.
Examples:
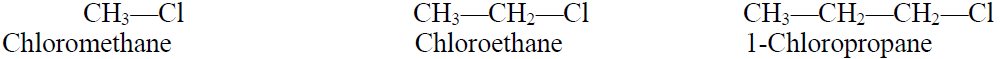
2- Secondary Alkyl Halides:
In secondary alkyl halides, the halogen atom is attached with a carbon which
is further attached to two carbon atoms.
Example:
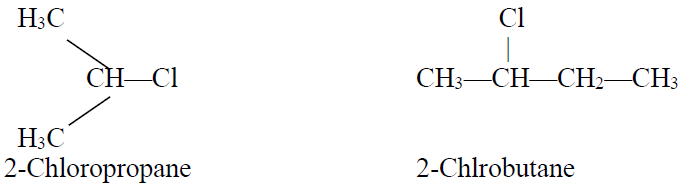
REACTIONS OF ALKYL HALIDES
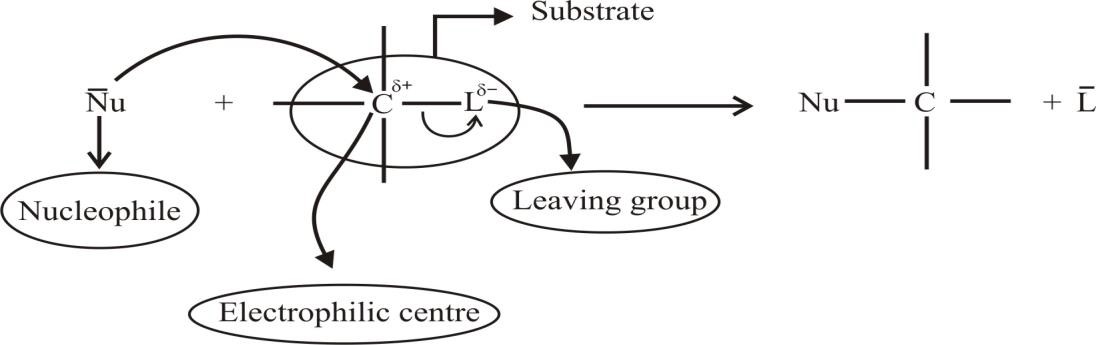
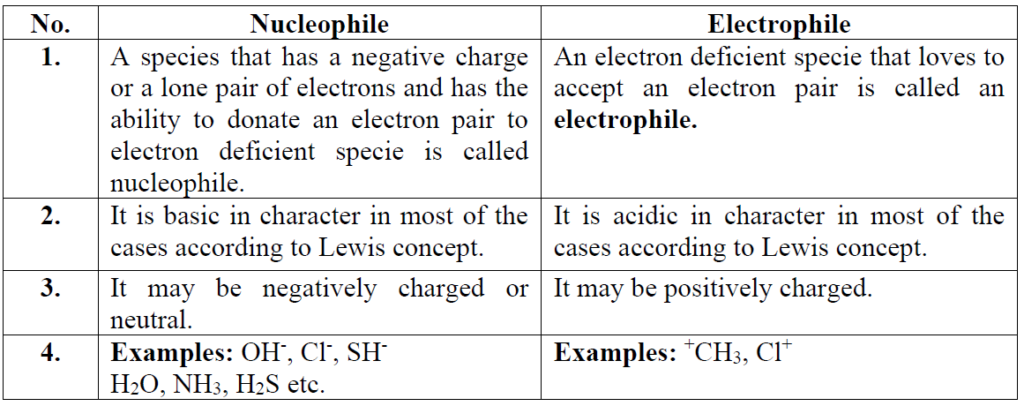
Q9. Define electrophile
Electron-deficient species may be positively charged or neutral
Example: +CH3, Cl+, BF3, AlCl3, SO3
Q10. Define nucleophile
Electron rich species may be negatively charged or neutral
Example: OH–, Cl–, SH–, H2O, NH3, H2S.
Q11. Define leaving Group
Halogen atom that leaves molecule on attack of attacking nucleophile.
Example: Cl– Br– I– OH–, -OR, -NH2
Q12. Define Substrate molecule.
Alkyl halide molecule on which nucleophile attacks
MECHANISM OF NUCLEOPHILIC SUBSTITUTION REACTIONS
Q13. Write a brief note on Nucleophilic substitution bimolecular reaction (SN2)
The arrival and departure of nucleophiles take place simultaneously.
1- Introduction
Single step mechanism. Primary alkyl halides give this reaction.
2- Direction of attack of Nucleophile
Attacking nucleophile attacks from the opposite side of the leaving group.
3- Variation in hybridization
sp3 to sp2
4- Mechanism of reaction:
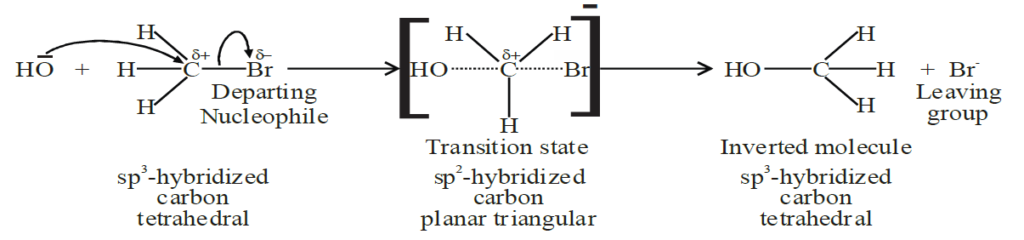
5- Inversion of configuration of molecule:
100% inversion.
6- Molecularity:
Two
7- Rate of reaction:
Rate = k [alkyl halide]1 [Nucleophile]1
8- Order of reaction:
Two
Q14. Write a brief note on Nucleophilic substitution unimolecular reaction (SN1)
1- Introduction:
Two-step mechanism. Tertiary alkyl halides give this reaction
Step 1:
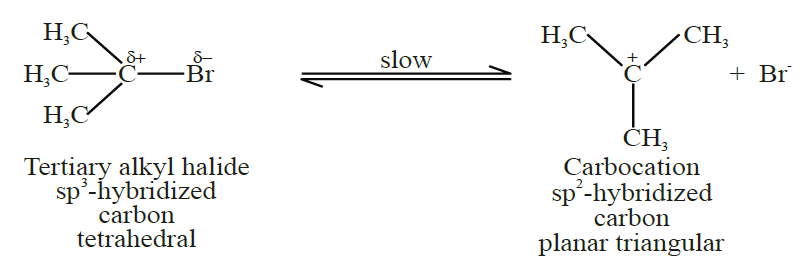
Step 2:
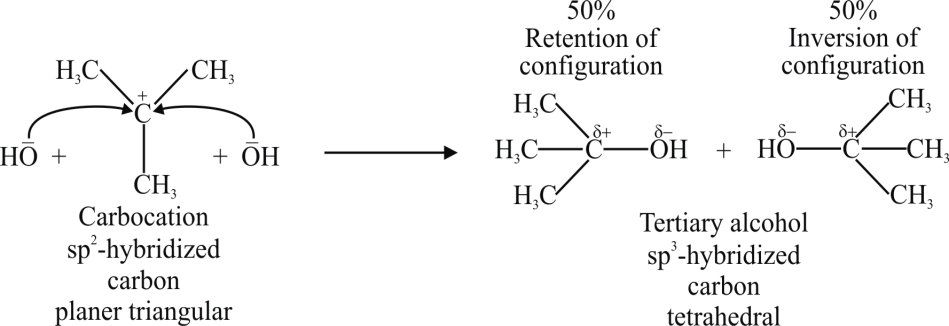
2- Molecularity:
One
3- Rate of Reaction:
Rate = k [alkyl halide]1
4- Order of Reaction:
One
Example of Nucleophilic Substitution reactions. :

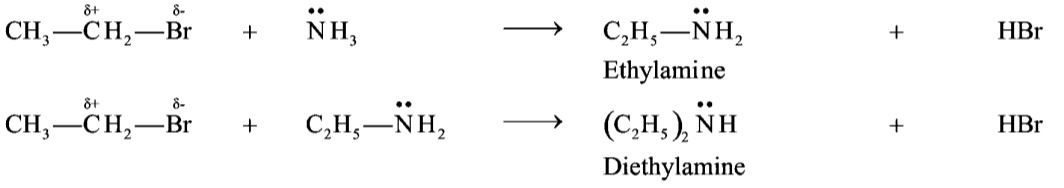
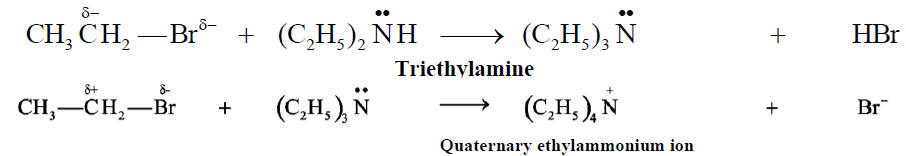
Q15. Define nucleophile. Give two examples.
The reagent that possesses an electron pair to form a covalent bond is called a nucleophile. These species are either negatively charged Example: OH– or neutral Example: NH3. The nucleophiles are further divided into the following groups.
- Strong Nucleophiles (-OH, -OR, NH3) Poor leaving groups.
- Weak Nucleophiles (Cl–, Br–, I– , HSO4–) Good leaving Groups
Furthermore, a leaving group is also a nucleophile but it is weaker than the attacking nucleophile but Iodide ion is a good nucleophile and as well as a good leaving group.
Q16. Differentiate between nucleophile and electrophile.
β-ELIMINATION REACTIONS
Q17. Write a brief note on β-ELIMINATION BIMOLECULAR REACTION (E2).
1- Introduction
Single-step reaction. Primary alkyl halides give this reaction.
2-Mechanism
Attack of nucleophile on β-hydrogen, breakage of C-X bond, formation of π-bond
between α- α-carbon and β-carbon.
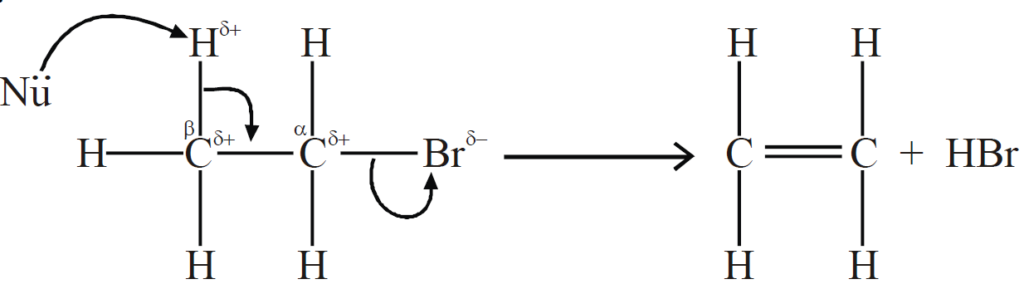
3- Hybridization
sp3 to sp2
4- Molecularity:
Two
5- Rate of reaction
Rate = k [alkyl halide]1 [Nucleophile]1
6- Order of reaction:
Two
Q18. Write a brief note onβ-elimination unimolecular (E1)
1- Introduction
Two-step reaction. Tertiary alkyl halides give this reaction.
Step1:
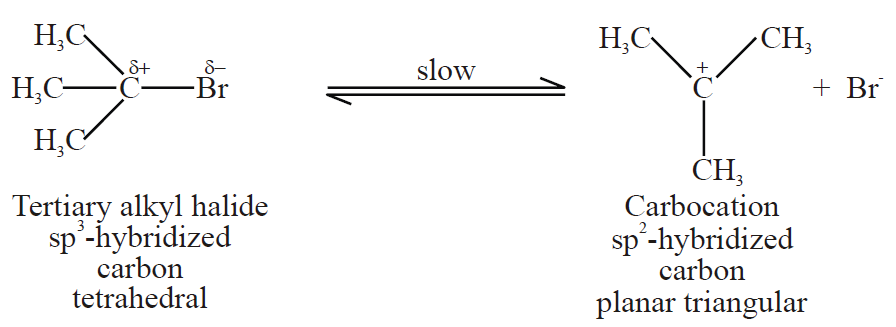
Step2:
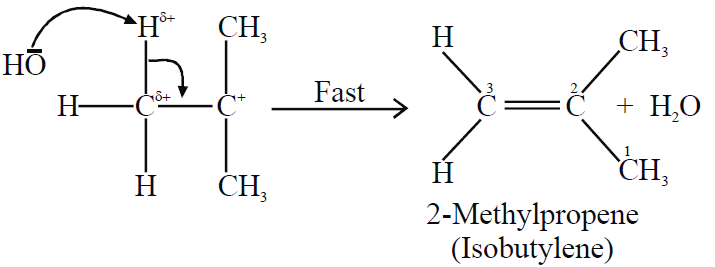
2. Molecularity:
One
3- Rate of reaction
Rate = k [alkyl halide]1
4- Order of reaction:
One
GRIGNARD REAGENT
Q19. Write a Brief note on Grignard’s Reagent.
They are alkyl magnesium halides. They are derivatives of alkyl halides belonging to the class of organo-metallic compounds.
General formula: R-Mg-X
Preparation of Grignard Reagent
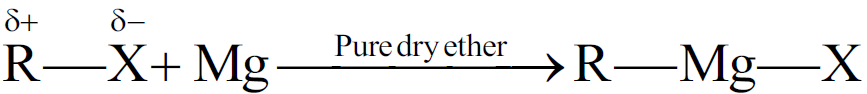
Reactivity order of alkyl halides with magnesium for a given alkyl group.
R — I > R — Br > R—Cl > R — F
Reactivity order of alkyl halide with magnesium for a given halogen.
CH3 — X > C2H5 — X > C3H7—X
Structure of Grignard Reagent
The magnesium atom is bonded to the alkyl group and a halogen atom.
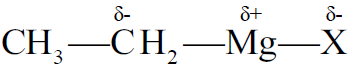
Reactivity
It depends upon the C-Mg bond polarity.
Reactions:
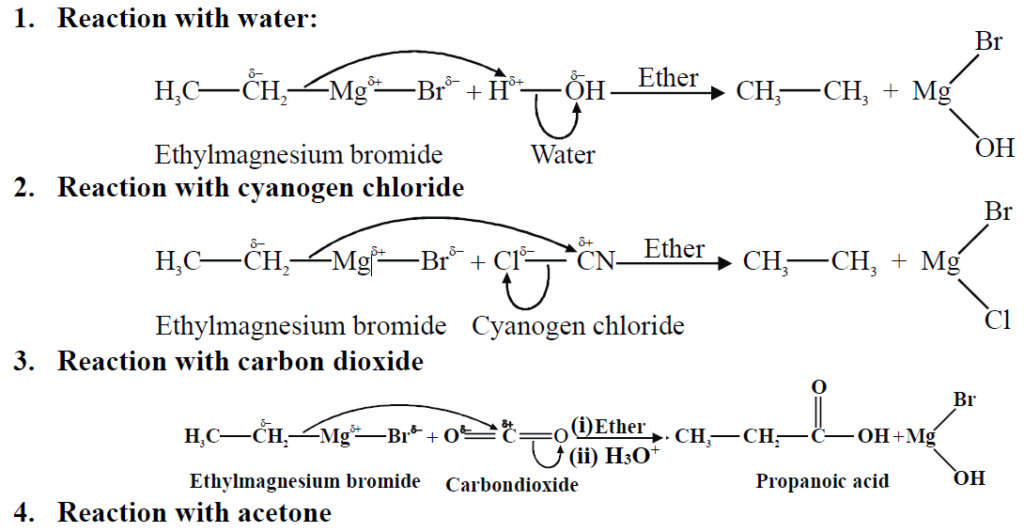
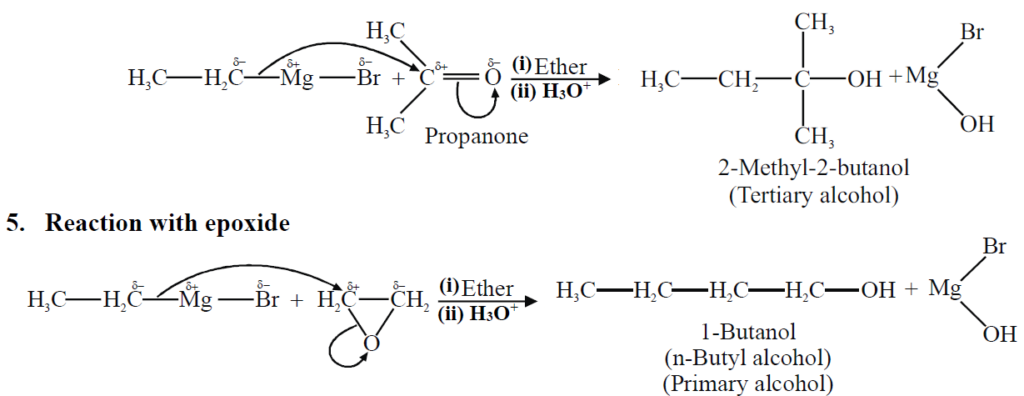
Q20. Describe Wurtz Synthesis for the preparation of alkanes.
Alkyl halide reacts with sodium in ether solvent to give alkanes. This reaction is particularly useful for the preparation of symmetrical alkanes. Unsymmetrical alkanes cannot be prepared due to a large number of side products.