Chemical equilibrium is an important chapter as it includes many solid concepts to understand. Umair Khan Academy provides notes along with video lectures. For all notes visit Umair Khan Academy.
Notes including all Sort questions, are given below. Long questions can be answered by combining a few short questions.
REVERSIBLE AND IRREVERSIBLE REACTIONS
Q1. Define chemical reaction. Give its types.
A process in which substances undergo a change in chemical composition
Example: Na + H2O → NaOH + H2
Types of Chemical Reactions:
- Irreversible chemical reaction:
A chemical reaction which proceeds in one direction only
Example: Na + H2O → NaOH + H2 - Reversible Chemical Reaction:
A chemical reaction that proceeds in both directions i.e. forward & reverse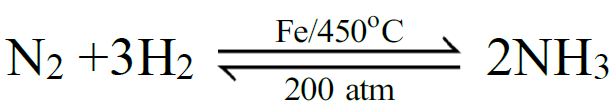
STATE OF CHEMICAL EQUILIBRIUM
Q2. Explain the state of Chemical Equilibrium.
Definition
A state during a reversible chemical reaction at which
- Rates of two opposing reactions become equal
- The concentration of reactants and products that become constant is called a chemical equilibrium.
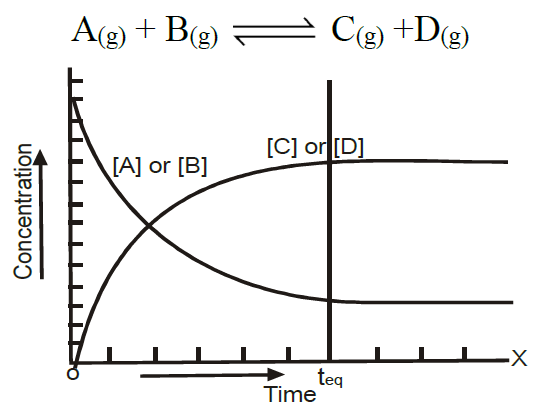
- Chemical Equilibrium is a Dynamic Equilibrium:
In which two opposing processes are proceeding at an equal rate - Characteristics of Chemical Equilibrium:
At the state of equilibrium
- The rate of forward and backward reactions becomes equal.
- The concentration of reactants and products becomes constant.
- The reaction does not cease but proceeds in both directions at the same rate.
- This state is attained for the reversible reaction no matter in which direction the reaction is
proceeding. - At equilibrium, both reactants and products are present in a definite ratio.
Example:
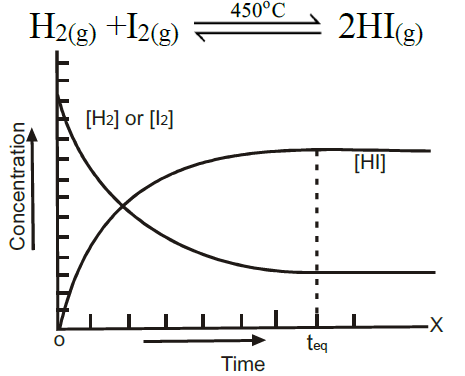
Q3. State the Law of Mass Action.
The rate of reaction is directly proportional to the product of active masses of reacting species.
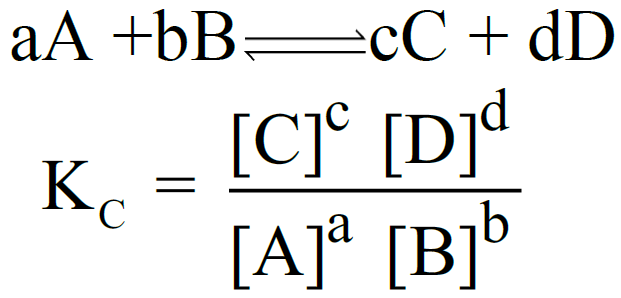
Q4. Write down the Units of Equilibrium Constant (Kc).
- Kc has no units when the number of moles of reactant & product are equal.
The unit of Kc depends upon the number of moles of reactants and products.
Example:
- KC have certain units when no. of moles of reactants and products are not equal.
Example: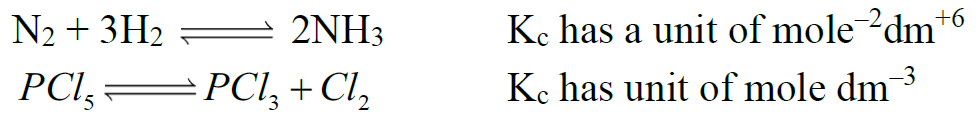
Q5. What are the units of Kc for a given reaction?
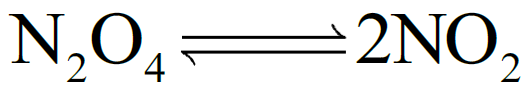
According to the law of mass action.
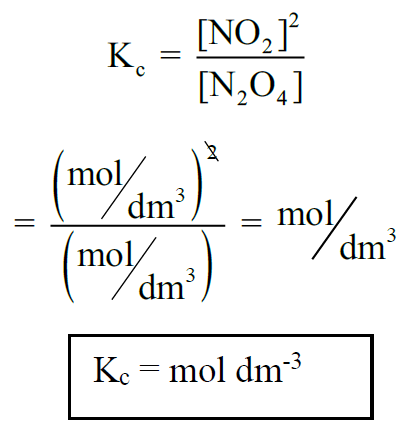
Q6. Write equilibrium constant expression for some important reactions.
1- Formation of an Ester: (Aqueous phase reaction)
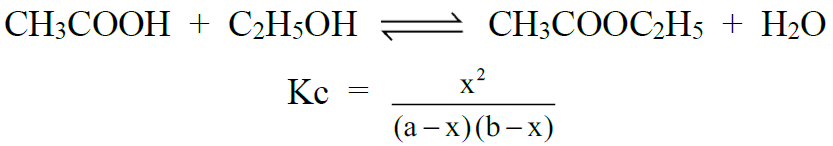
2- Dissociation of PCl5: (Gaseous phase reaction)
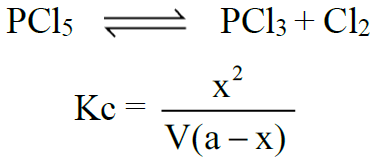
Q7. Write down relationships between equilibrium constants (Kp And Kc).
Let us consider the following general reaction.
aA + bB ⇌ cC + dD
The equilibrium constant is called Kc
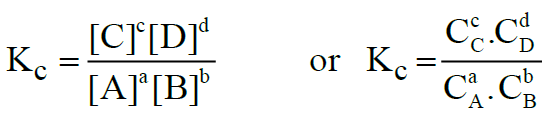
The equilibrium constant is called Kp
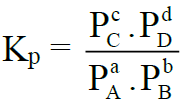
Relationship between Kp and Kc
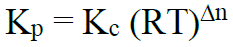
Δn = no. of moles of products – no. of moles of reactants
Q8. Write the applications of the equilibrium constant.
1- Direction of Reaction:
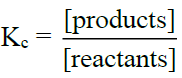
- a) If [Products]/[Reactants] < Kc then the reaction will proceed (forward)
- b) If [Products]/[Reactants] > Kc then the reaction will move backwards (reverse)
- c) If [Products]/[Reactants] = Kc then it means the reaction is already at equilibrium.
2- Extent of Reaction:
- a) If the value Kc is very large: the reaction is almost complete
- b) If the Kc value is small: the reaction does not proceed (moves forward) appreciably
- c) If the value of Kc is very small this shows a very little forward reaction.
3- The Effect of Conditions on The Position of Equilibrium:
- Kc is equilibrium constant, it remains constant for a particular reaction at a constant temperature
- It can be varied if external conditions like temperature, pressure and concentrations are altered.
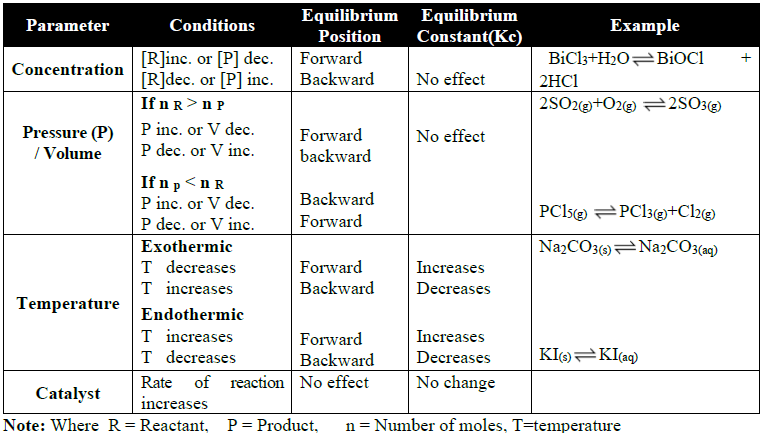
THE LE-CHATELIER’S PRINCIPLE
Q9. The Le-Chatelier’s Principle.
Statement:
If stress is applied to a system at equilibrium then the system acts in such a way that it nullifies the effect of the stress as far as possible.
Effect of Change in Different Parameters:
Q10. Write down the applications of chemical equilibrium in industry.
1- Synthesis of Ammonia by Haber’s Process:
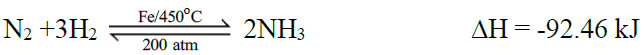
Maximum yield of ammonia may be obtained by
- continual removal of ammonia
- increase in pressure
- decrease in temperature
2- Preparation of Sulphur Trioxide:

Maximum yield of sulphur Trioxide may be obtained by
- continual removal of SO3
- decreasing temperature
- increasing pressure
IONIC PRODUCT OF WATER
Q11. Write the equilibrium constant expression for the ionic product of water.
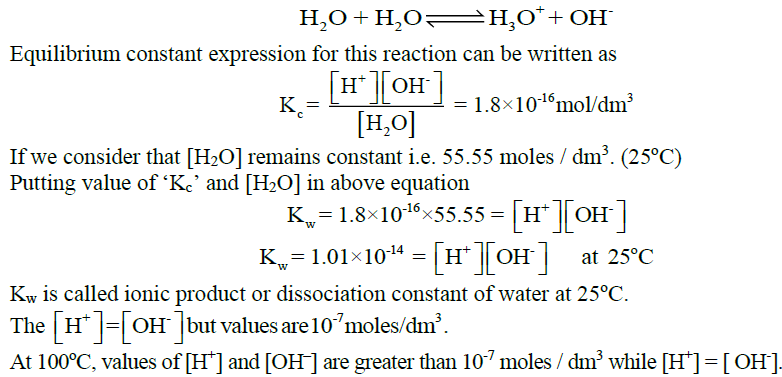
Q12. Write Relation between Kw and Temperature:
By increasing the temperature Kw also increased. (Kw ∝ T)
IONIZATION CONSTANT OF ACID (Ka)
Q13. Describe the ionization constant of acid (Ka)
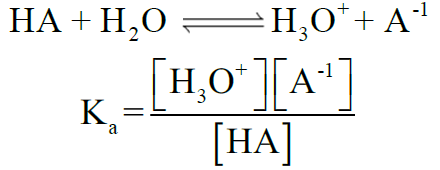
Ka for Acidic Strength:
Ka < 10-3 acid is weak
Ka = 1 to 10-3 acid is moderately strong,
Ka > 1 acid is strong
Percentage of Ionization of Acids:
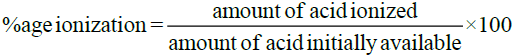
IONIZATION CONSTANT OF BASES (Kb)
Q14. Describe ionization constant of bases (Kb)
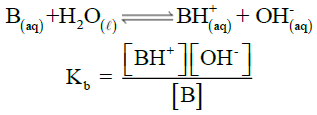
LOWRY–BRONSTED CONCEPT OF ACIDS AND BASES
Q15. Write Lowry Bronsted concept of acids and bases
- The species which release a proton or have a tendency to release a proton in water are called
acids. - The species which accept a proton or have a tendency to accept proton in water are called
bases.
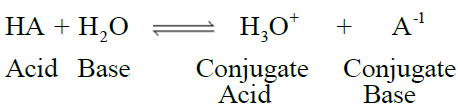
Q16. Write relations of pKa, pKb, and pKw
pKa + pKb = pKw
As pKw at 25 oC is 14,
So, pKa + pKb = 14
Q17. Calculate the pH of 10-4 moles/dm3 of HCl.
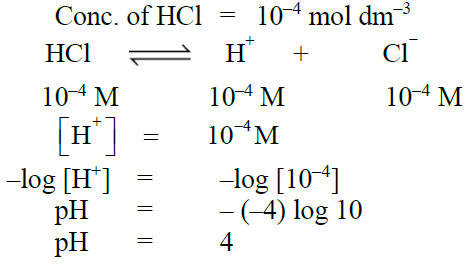
COMMON ION EFFECT
Q18. Define the Common Ion Effect.
Definition:
The suppression of ionization of weak electrolytes by strong electrolytes having common ion
Example:
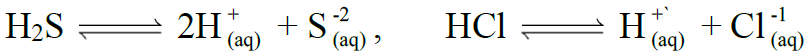
BUFFER SOLUTIONS
Q19. Write a brief note on Buffers.
Buffers
The solution which resists the change in its pH when a small amount of an acid or base is added is called a Buffer solution.
How do Buffers Act?
Buffer action can be explained by keeping in view the concepts of the common ion effect and Le-Chatelier’s principle.
Henderson’s Equation for the preparation of Buffer Solution of Required pH Value:
(Calculation of pH of a Buffer)

Buffer Capacity
resistance offered by the buffer to change in its pH
SOLUBILITY PRODUCT (Ksp)
Q20. Define Solubility Product. Give its applications.
The product of equilibrium concentrations of ions of sparingly soluble salt is called solubility product.
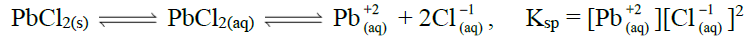
Applications of Solubility Product:
Following are the applications of solubility products.
- Determination of Ksp from solubility.
- Determination of solubility from Ksp
- Effect of common ion on solubility.
Ksp > Ionic Product ———— Unsaturated solution
Ksp = Ionic Product ———— Saturated solution
Ksp < Ionic Product ———— Super Saturated solution
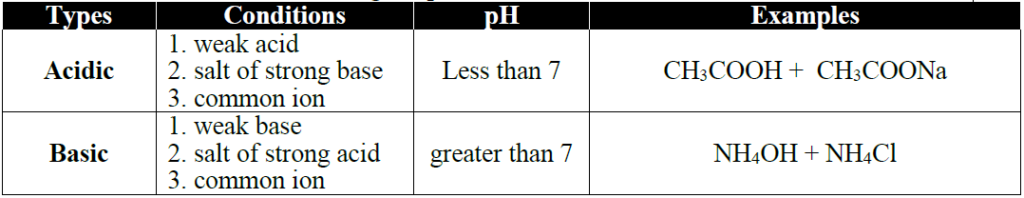
Q21. What are buffer solutions? How acidic buffer can be prepared?
Ans: The solutions which show resistance to change in their pH when a small amount of an
acid or base is added are called Buffer solutions.
There are two types of Buffer solutions
- Acidic Buffers
- Basic Buffers
Acidic buffer can be prepared by mixing weak acid and its salt with a strong base.
Example:CH3COOH + CH3COONa
Q22. What is the effect of common ions on solubility? Give example
When HCl gas is passed through the saturated solution of NaCl. Sodium chloride is crystallized out due to the common ion effect because HCl is more soluble in water than NaCl, so it will suppress the solubility of NaCl in the solution.
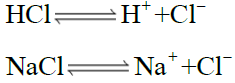
GET IN TOUCH
Visit YouTube Channel

Thanks Sir Ji 😇😇😇