‘Aldehyde and Ketones’ is an essential chapter including the carbonyl group. A quick revision notes are as follows. Short and long questions are written by keeping the examination point of view. Detailed answers can be written by joining a few headings. For more notes and help visit Umair Khan Academy.
INTRODUCTION
Q1. Define Carbonyl Compounds.
The organic compounds containing ![]() group.
group.
- Aldehydes:
The compounds in which the carbonyl group is bonded to at least one hydrogen atom are called aldehydes.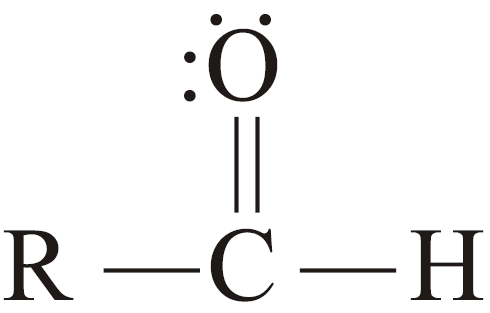
- Ketones:
In ketones, the carbonyl group is bonded to two carbon atoms, and so the carbonyl group occurs
within the chain.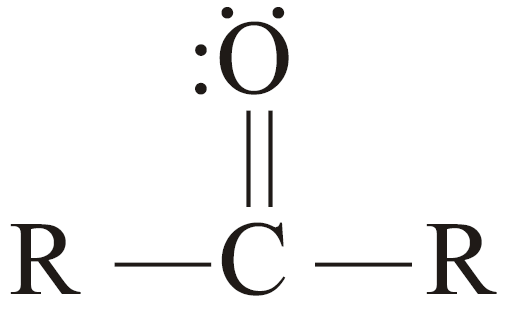
NOMENCLATURE
Q2. Give a very brief description of the common and IUPAC nomenclature of aldehyde.
- Common Names:
The common names of Aldehydes are obtained from the common names of carboxylic acids
containing the same number of carbon atoms. The ending-ic acid in the common name of the
acid is replaced by the word aldehyde.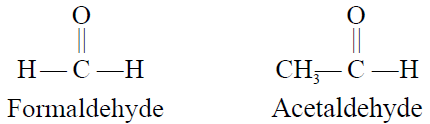
- IUPAC Names:
The IUPAC names of aldehydes are derived from the names of alkanes having the same number
of carbon atoms. The letter -e in the name of the alkane is replaced with al.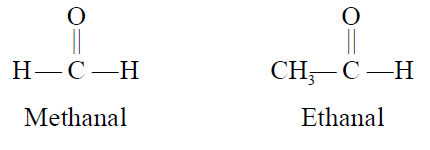
Q3. Give a very brief description of the common and IUPAC nomenclature of aldehyde.
- Common Names:
The common names of ketones are obtained by separately writing the names of the alkyl groups
attached to the carbonyl carbon. The word ketone is then added at the end as a separate word. The names of the alkyl groups are written alphabetically. When two alkyl groups are the same, the prefix di- is added before the name of the alkyl group.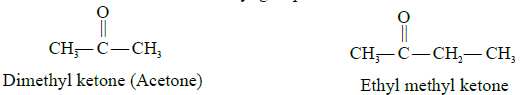
- IUPAC Names:
The IUPAC names of ketones are derived from the names of alkanes having the same number of
carbon atoms. The letter ‘e’ in the name of alkane is replaced with the suffix-“one”.
PREPARATION OF ALDEHYDES AND KETONES
Q4. Write down the preparation of Formaldehyde (Formalin)
- Laboratory Method
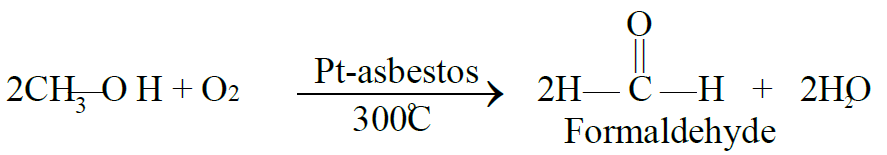
The resulting mixture is called formalin.
Formalin is 40% formaldehyde, 8% methyl alcohol and 52% water.
- Industrial Method
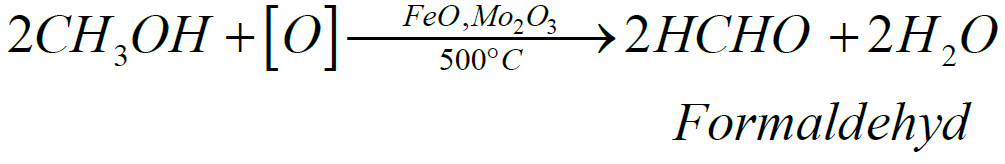
Q5. Write down the Preparation of Acetaldehyde.
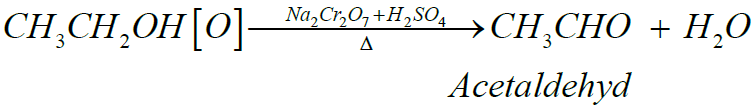
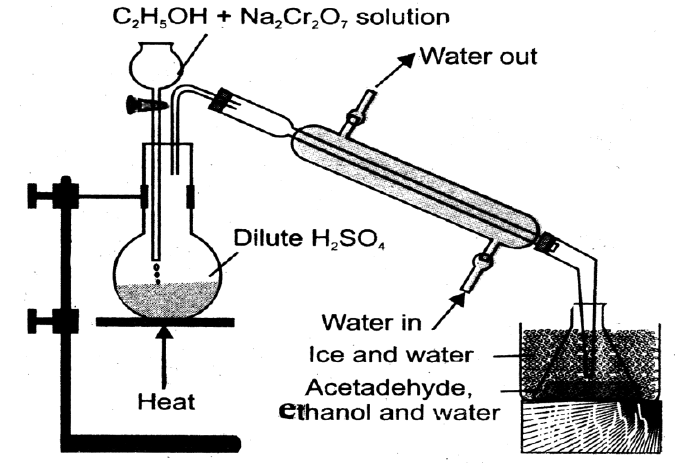
- Laboratory method: (Dry Distillation)
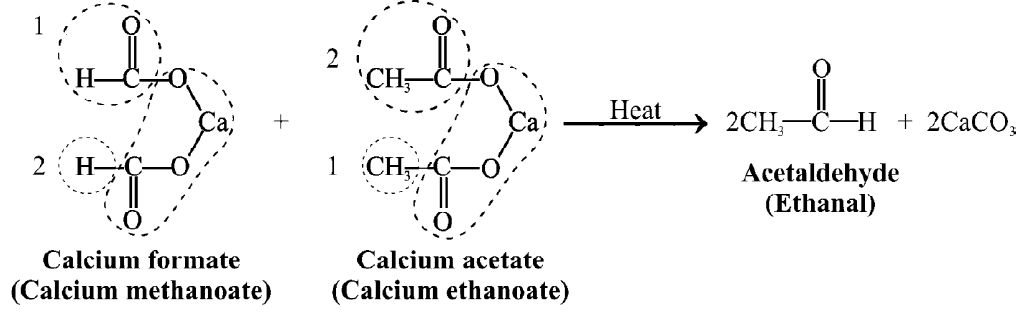
- Industrial methods:
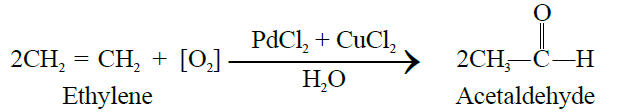
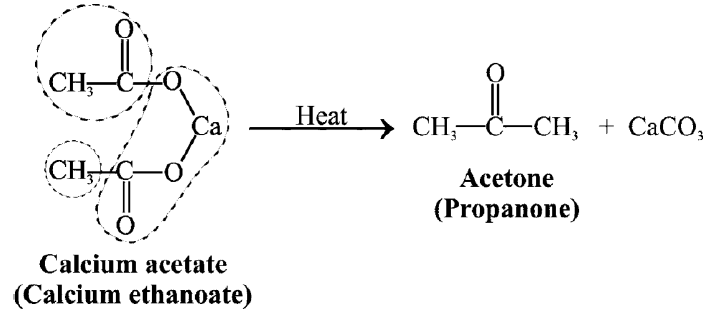
Q6. Write down the Preparation of Acetone.
REACTIVITY OF CARBONYL GROUP
Q7. Write a few lines on the reactivity of the carbonyl group.
In the carbonyl group, sp2 hybridized carbon is bonded to an oxygen atom through a σ-bond and a π-bond. Due to the presence of π-bond, it can undergo nucleophilic addition reaction.
Nucleophilic addition actions are of two types, Base catalysed reactions and Acid catalysed reactions.
Base-Catalyzed Addition Reactions
Q8. Write down the Base-Catalyzed Addition Reactions of carbonyl compounds.
A base-catalyzed nucleophilic addition reaction will take place with a strong nucleophilic reagent. The base reacts with the reagent and generates a stronger nucleophile.
General mechanism:
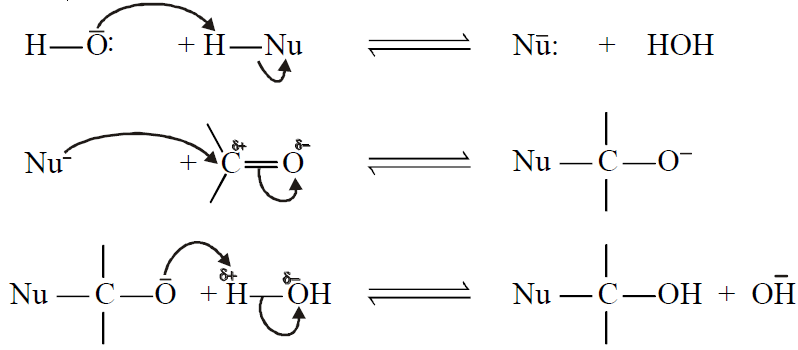
1- Addition of Hydrogen Cyanide:
Addition to Acetaldehyde
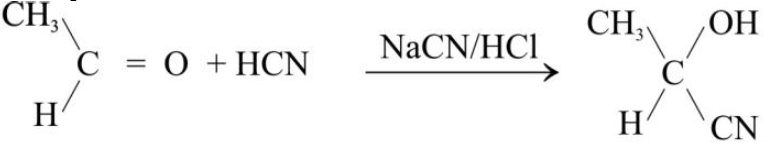
Addition to Acetone:
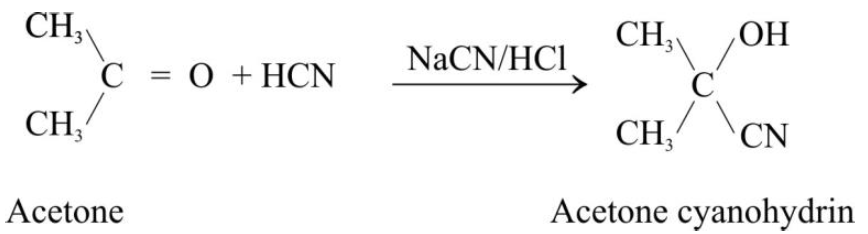
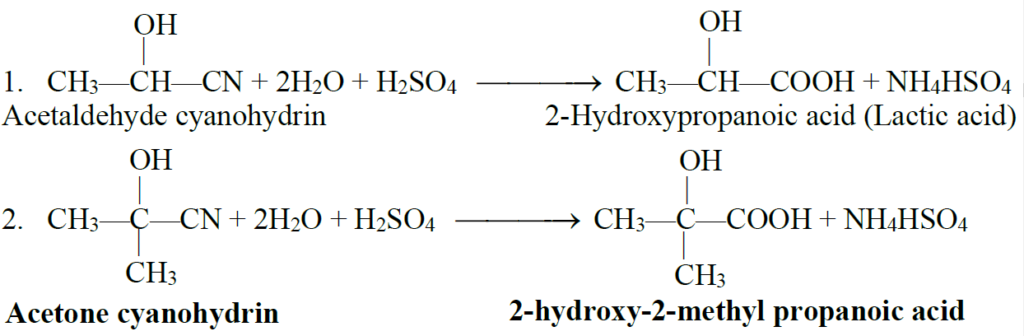
2- Hydrolysis of Cyano (—CN) group
3- Addition of Grignard Reagent.
Grignard reagent adds to aldehydes and ketones to form adduct which on hydrolysis with a dilute mineral acid (HCl, H2SO4) gives alcohols.
4-Addition of Sodium Bisulphite:
Addition to Formaldehyde:
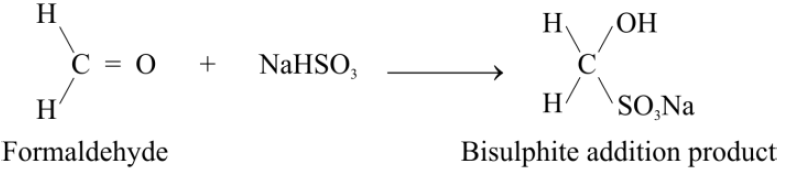
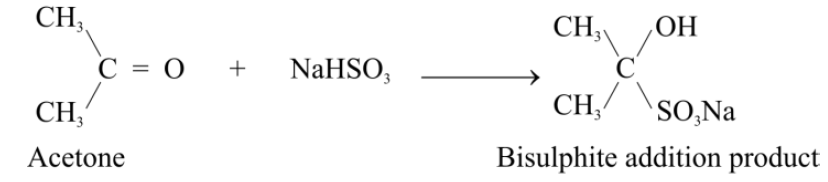
Addition to Acetone:
5- Condensation Reactions:
In which two molecules of the same or different compounds combine to form a new compound
with or without the elimination of a small molecule like H2O or NH3. i.e. Aldol condensation.
Q9. EXPLAIN THE ALDOL CONDENSATION REACTION.
Aldehydes and ketones possessing α-hydrogen atoms react with a cold dilute solution of an alkali to form addition products known as aldols.
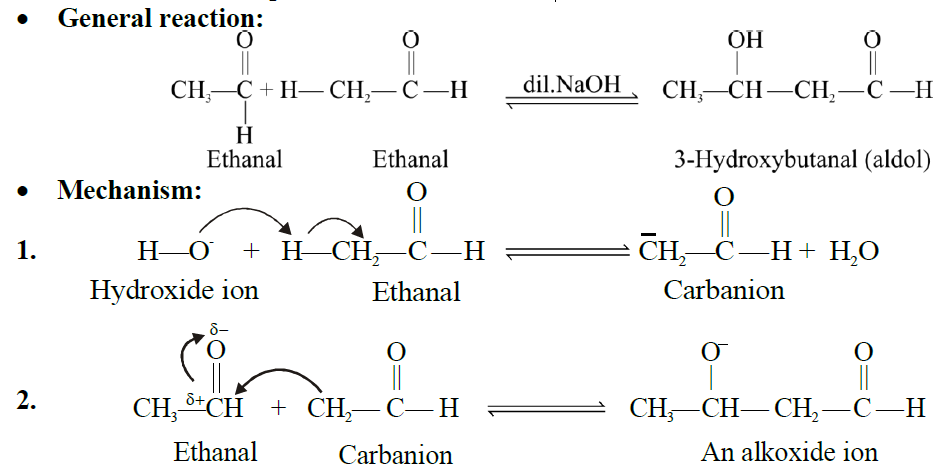
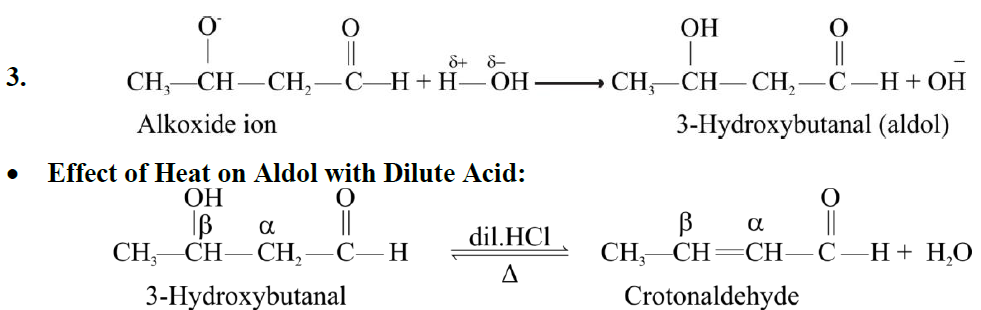
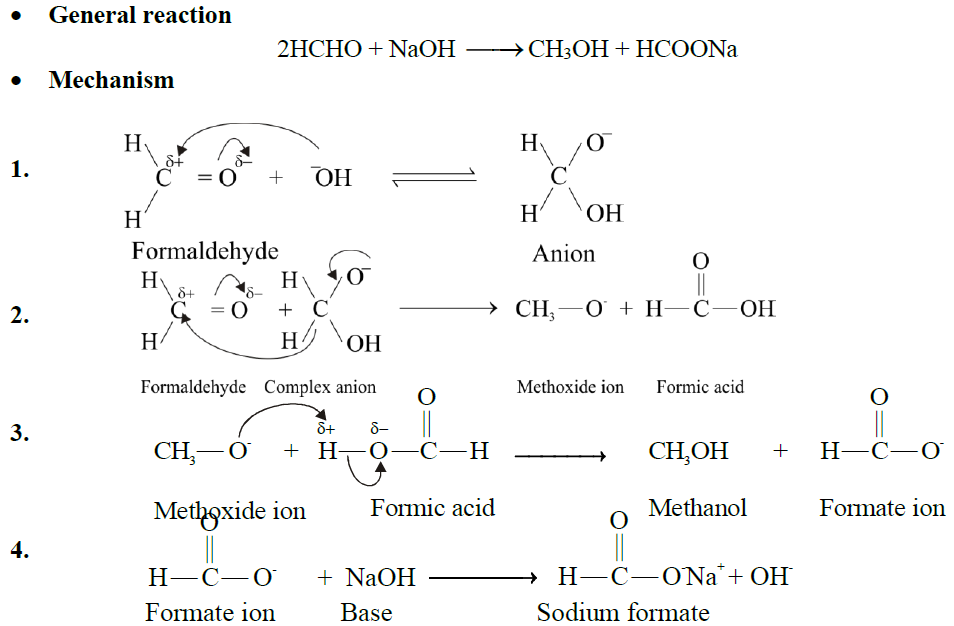
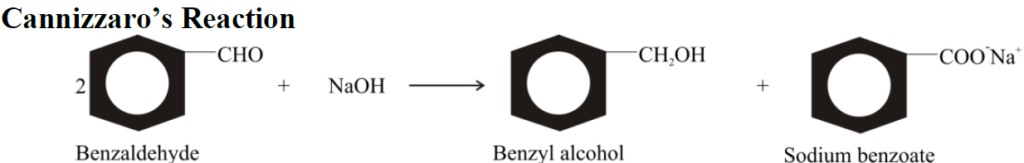
Q10. EXPLAIN CANNIZZARO’S REACTION:
Aldehydes that have no α-hydrogen atoms undergo Cannizzaro’s reaction. It is a disproportionation (self-oxidation-reduction) reaction.
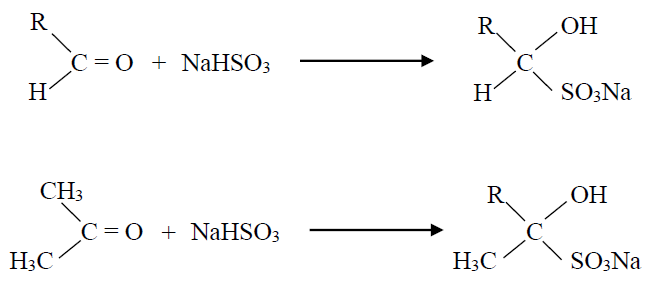
Q11. What is the sodium bisulphite test?
Almost all the aldehydes and methyl ketones give a crystalline white precipitate with a saturated solution of NaHSO3. This white precipitate is due to the formation of an adduct. This reaction can be used as a test to distinguish between methyl ketones and higher ketones.
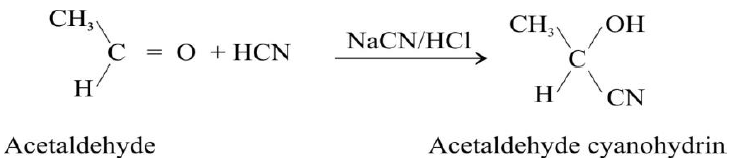
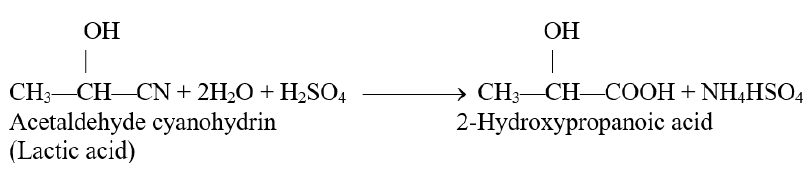
Q12. Convert acetaldehyde into lactic acid.
Acetaldehyde can be converted into lactic acid
a): by first adding HCN to acetaldehyde to get acetaldehyde cyanohydrin. This reaction is carried out by adding slowly mineral acid to an aqueous solution of sodium cyanide. The acid generates HCN from NaCN in situ.
b): acetaldehyde cyanohydrin on hydrolysis by an aqueous acid converts to lactic acid as follows,
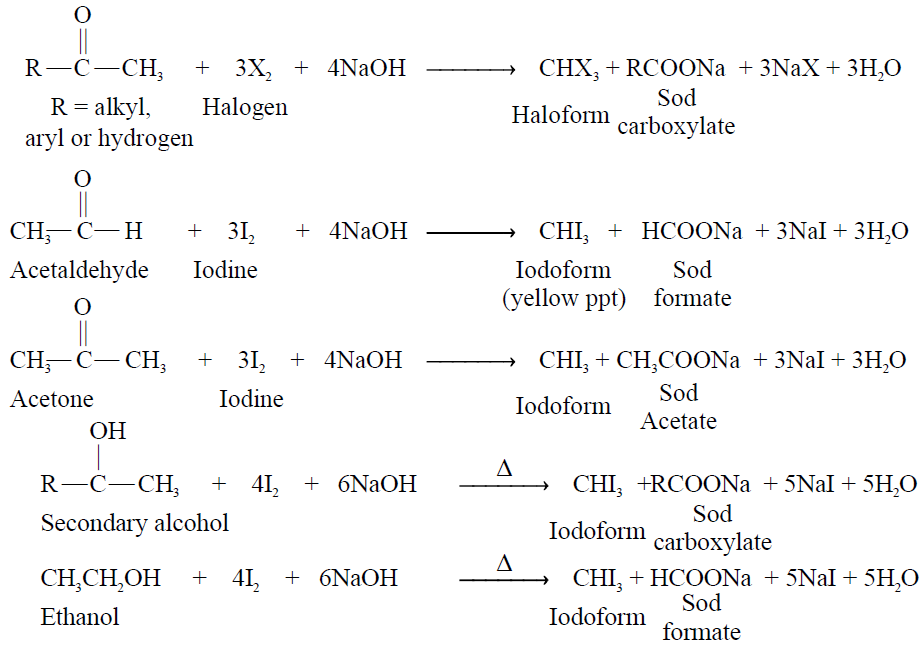
Q13. What are haloform reactions, give examples.
Definition:
In which acetaldehyde, methyl ketones or 2-alkanols react with halogen in the presence of sodium hydroxide
to give haloform and salt of acid. The compounds that give haloform reaction are:
- Only acetaldehyde (among aldehydes)
- Methyl ketones (among ketones)
- 2-alkanol (secondary alcohol) and only ethanol (among primary alcohol)
General Reaction.
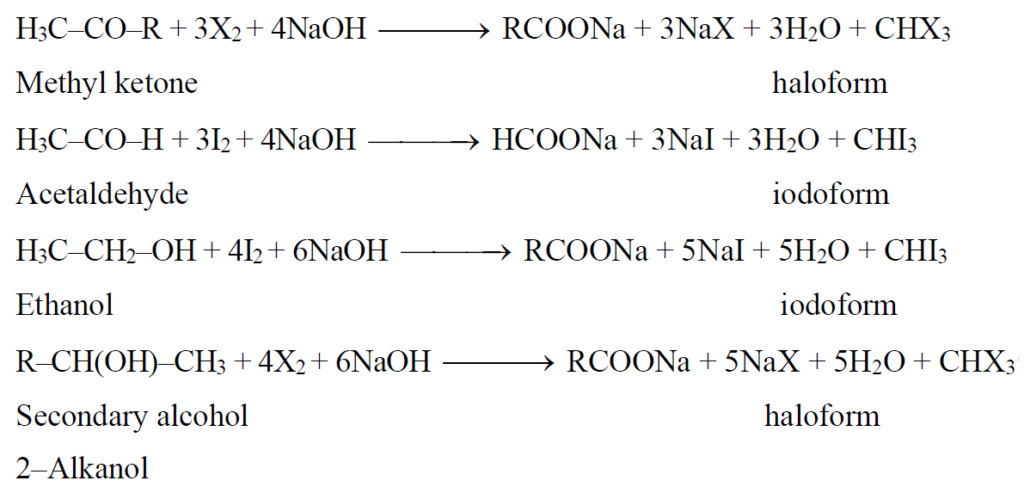
Q14. What is the Iodoform Test for and how it is carried out?
The reaction in which acetaldehyde, methyl ketones or 2-alkanols out of all secondary alcohols, react with halogen in the presence of sodium hydroxide to give haloform and salt of acid is called haloform reaction. The haloform may be Chloroform, Bromoform and iodoform. The compounds that give haloform reaction are:
- Only acetaldehyde (among aldehydes)
- Methyl ketones (among ketones)
- 2-alkanol (secondary alcohol) and only ethanol (among primary alcohol)
ACID-CATALYZED ADDITION REACTION
Q15. Write down a brief note on acid-catalysed addition reactions.
The acid-catalyzed nucleophilic addition reaction will take place with a weak nucleophilic reagent. The addition is initiated by the proton (H+) liberated by the acid.
The general mechanism of the reaction is as follows:
Q16. Write some examples of Acid-Catalyzed Nucleophilic Addition Reactions:
Polymerization:
- Formaldehyde:
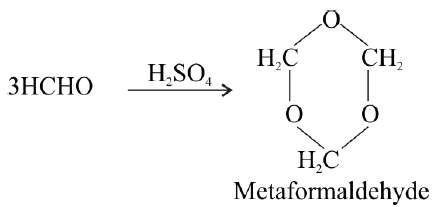
- Acetaldehyde:
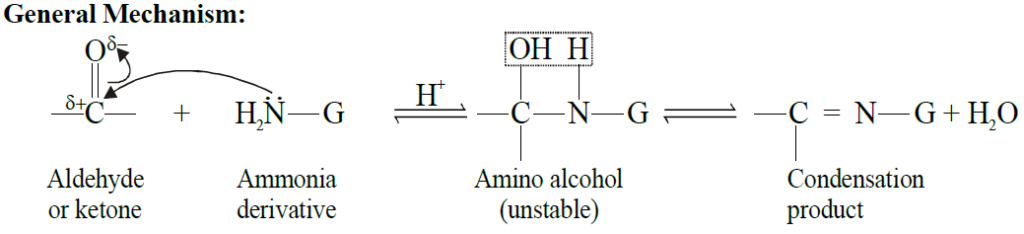
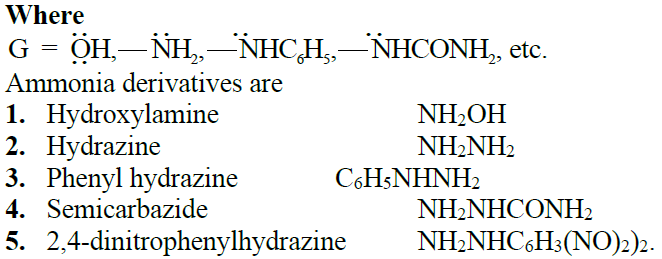
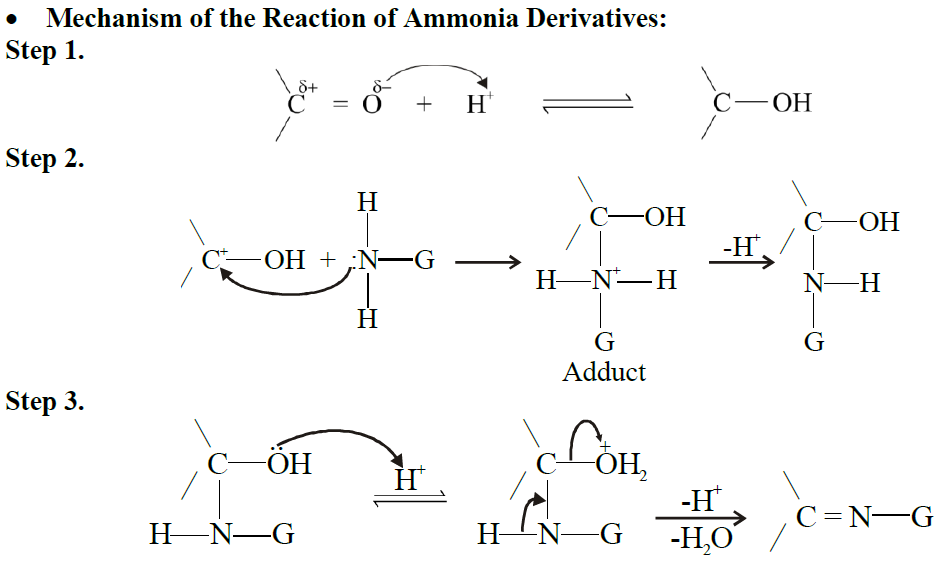
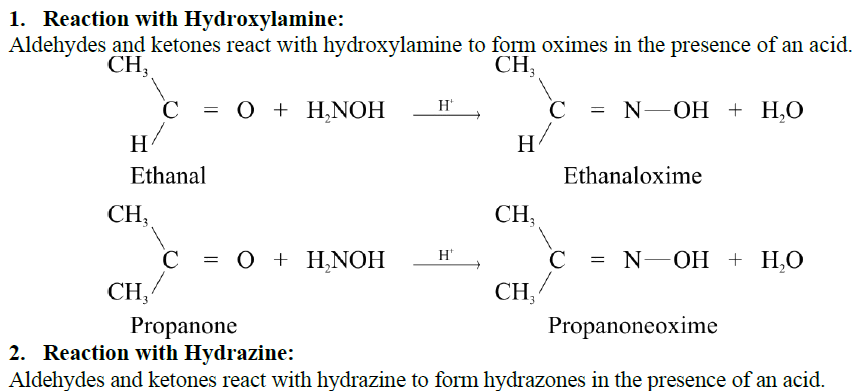
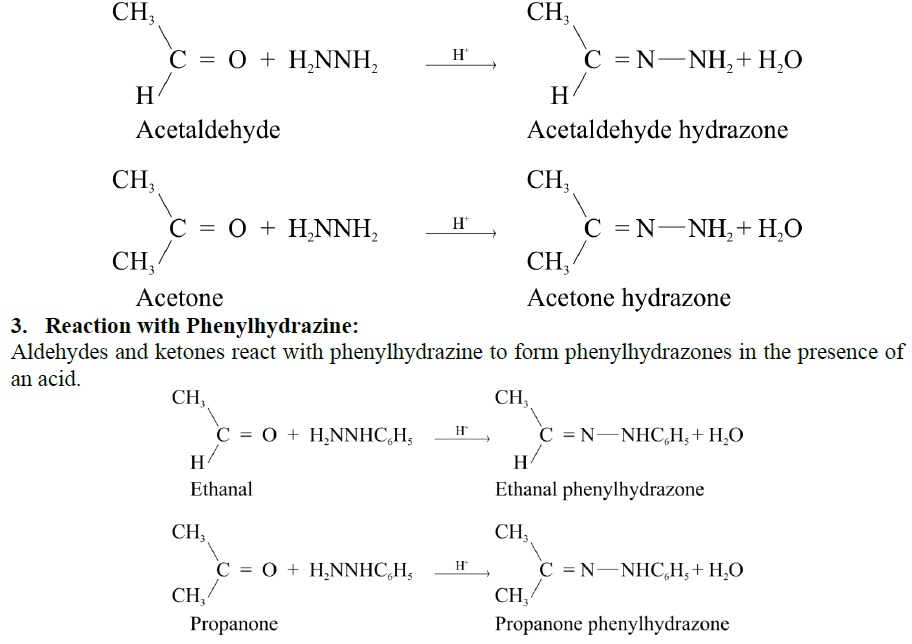
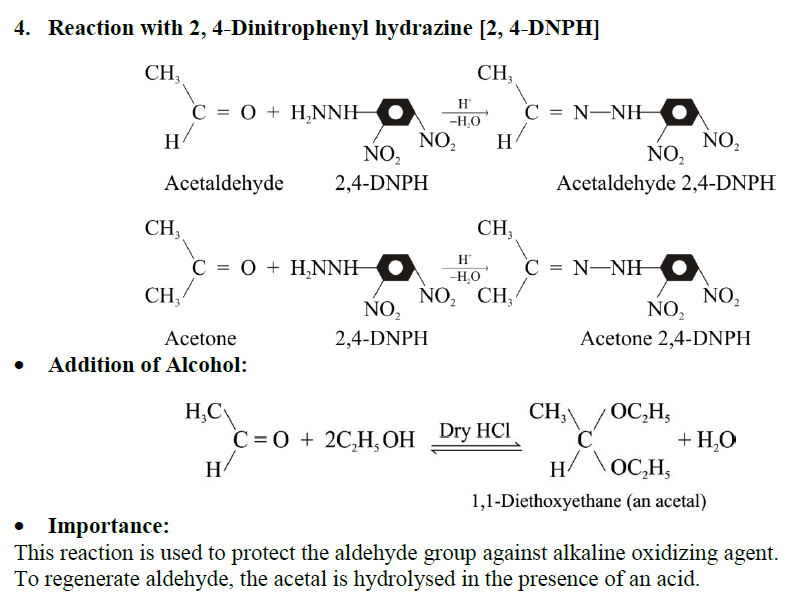
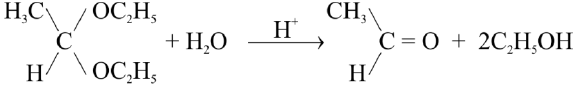
Q17. Write down the general mechanism of the addition of ammonia derivatives to aldehydes and ketones. Give important reactions.
Reduction Reactions of Adehyde and Ketones
Q18. Write down the Reduction reactions of aldehydes and ketones.
Reduction with sodium borohydride.
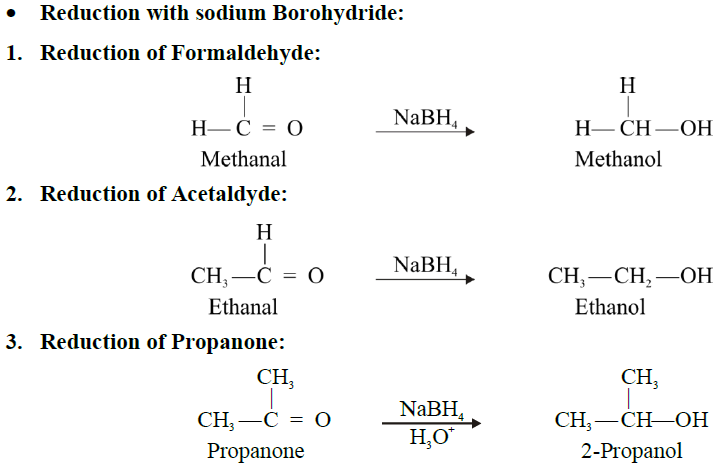
Catalytic reduction
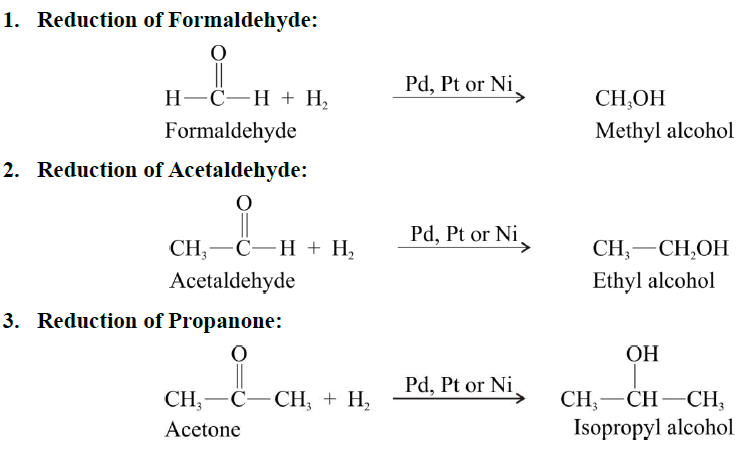
OXIDATION REACTIONS OF ALDEHYDE AND KETONES
Q19. Write down a brief note on the oxidation reactions of aldehyde and ketones.
Aldehydes are easily oxidized by mild oxidizing agents like Tollen’s reagent, Fehling’s solution and Benedict’s solution.
- They are oxidized to carboxylic acids by strong oxidizing agents such as K2Cr2O7 / H2SO4 or KMnO4 / H2SO4 and dilute nitric acid.
- The hydrogen atom attached to the carbonyl group in aldehydes is oxidized to the OH group. This is comparatively easier to oxidize.
- Hence, aldehydes can be oxidized by mild oxidizing agents.
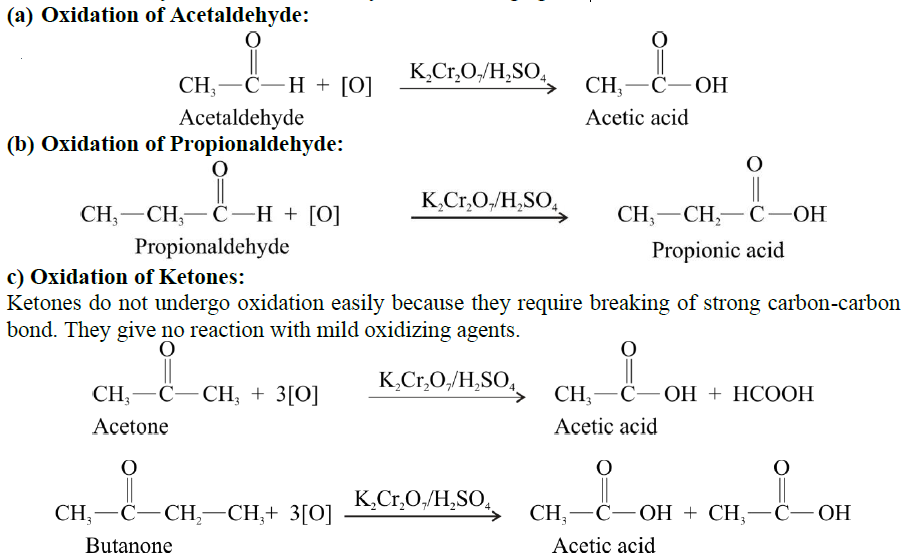
IDENTIFICATION OF CARBONYL COMPOUNDS
Q20. Write some identification tests for Aldehydes and ketones.


Q21. Write some uses of Formaldehyde and Acetaldehyde.
- Uses of formaldehyde
- In the manufacture of resins like urea-formaldehyde and plastics such as bakelite.
- In the manufacture of dyes such as Indigo, Para-rosaniline, etc.
- Its 40% aqueous solution called formalin is used as an antiseptic, a disinfectant, a germicide, a fungicide and for preserving animal specimens and sterilizing surgical instruments.
- As a decolourising agent in vat dyeing.
- In the silvering of mirrors.
- In making medicine urotropine is used as a urinary tract antiseptic.
- In the processing of anti-polio vaccine.
- In making firmament (formaldehyde + lactose) is used as throat lozenges.
- Uses of acetaldehyde
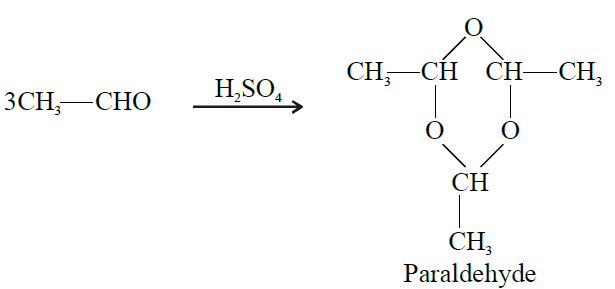
- In the production of acetic acid, acetic anhydride, n-butanol, ethanol, 2-Ethyl -1-hexanol, vinyl acetate, paraldehyde, ethyl acetate etc.
- To make acetaldehyde ammonia, used as a rubber accelerator.
- To make chloral hydrate, ethanol trimer and tetramer. Chloral hydrate and ethanol trimmer are both used as hypnotic drugs and ethanol tetramer is used as a slug poison.
- As an antiseptic inhalant in nasal infections.
- In silvering of mirrors.
- To make phenolic resins and synthetic drugs.
Q22. Give chemical changes in two steps that occur by the addition of Tollen’s reagent to an aldehyde. In a test tube and heated.
Aldehydes form silver mirror with Tollen’s reagent (ammoniacal silver nitrate solution). Add Tollen’s reagent to an aldehyde solution in a test tube and warm. A silver mirror is formed on the inner of the test tube.
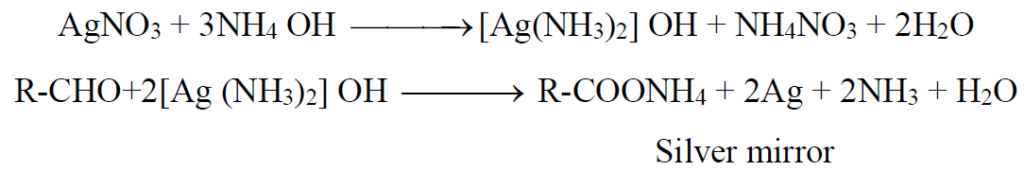
High-quality mirrors are manufactured by using this principle.
Q23. How an aldehyde is distinguished from a ketone by Fehling’s solution test? Write the equation as well.
Aliphatic aldehydes form a brick-red precipitate with Fehling’s solution. To an aldehyde solution, add Fehling’s solution and boil. A brick-red precipitate of cuprous oxide is formed.

Ketones do not give this test.
