Umair khan academy is providing the valuable notes for its students. Thermochemistry is about the heat changes during the Chemical reaction. Chapter is written in terms of short questions, which is helpful for exams preparation.
Introduction to Thermochemistry
Q. Define thermochemistry.
Study of heat changes during a chemical reaction.
Q. Define Heat of Reaction
Energy is absorbed or released in a chemical reaction is called heat of reaction.
Q. Write the types of thermochemical reactions.
There are two types of such reactions.
- Exothermic Reaction
Reaction in which energy is released is called exothermic reaction. Heat evolved is expressed with negative sign.
Example: C(s) + O2 (g) → CO2 (g) ΔH = – 393.7kJ mol-1 - Endothermic Reaction
Reaction in which energy is absorbed is called endothermic reaction. Heat absorbed expressed with positive sign.
Example: 2H2O(l) → 2H2(g) +O2(g) ΔH = +285.58kJ mol
Q. What are the Units of energy in SI-system
Joule (J) and kilo Joule (kJ).
Q. What is the Importance of Thermochemistry?
Gives information about heat contents of compounds, which helps study of chemical bonding and chemical equilibrium.
Q. What are the Limitation of Thermochemistry
Only few chemical reactions whose heats of reaction can be accurately measured can be studied.
SPONTANEOUS AND NON-SPONTANEOUS REACTIONS
Q. Define Spontaneous Reaction:
The reaction which Takes place on its own without any outside assistance is called spontaneous reaction.
Example:
- Flow of water from higher level to lower level.
- NaOH(aq) + HCl(aq) ⇌ NaCl(aq)+ H2O(l)
- A reaction will also be called a spontaneous reaction, if it needs energy to start but proceeds further on its own for example burning of coal:
Q. Define Non-Spontaneous Reaction:
The reaction which does not take place on its own without any external aid is called non-spontaneous reaction. Example: Pumping of water uphill.
SYSTEM, SURROUNDINGS AND STATE FUNCTIONS
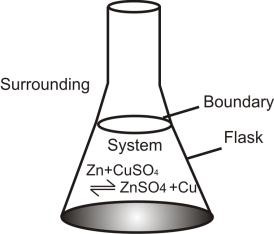
Q. Define System:
Any portion of universe which is under study (consideration).
Q. Define Surrounding:
Portion of the universe other than system.
Q. Define Boundary:
The real or imaginary surface separating the system from surroundings.
Examples:
Consider, the reaction between ‘Zn’ and CuSO4 solution. This can be called a system because it is under observation. The flask, the air etc. are the surroundings.
Q. Define State of System
- Initial State of a System:
A state showing Initial conditions of the system before any change is called initial state of the system. - Final State of a System:
A state showing final conditions of the system after any change is called final state of the system.
Q. Define State function.
A macroscopic property of a system which has some definite values for initial and final states and is independent of the path adopted to bring about the change.
Example:
- Change of temperature = ∆T = T2 – T1
- Change of enthalpy = ∆H = H2 – H1
- Change of internal energy = ∆E = E2 – E1
INTERNAL ENERGY (E)
Q. Define Internal Energy.
The sum of the kinetic as well as the potential energies of system is called internal energy of system.
Q. What are the basic kinds of internal energies?
There are two types.
- Kinetic Energy
Kinetic energy accounts for- Translational motion
- Vibrational motion
- Rotational motion
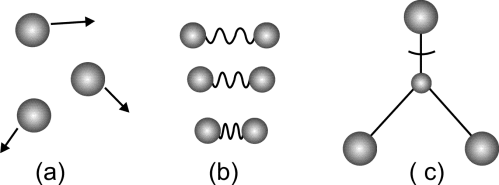
- Potential energy
Potential energy accounts for- Intramolecular forces
- Intermolecular forces
Q. Define Heat (q).
The quantity of energy that flows across the boundary of a system.
Signs of Heat
- Heat absorbed by the system is +ve.
- Heat evolved by the system is -ve.
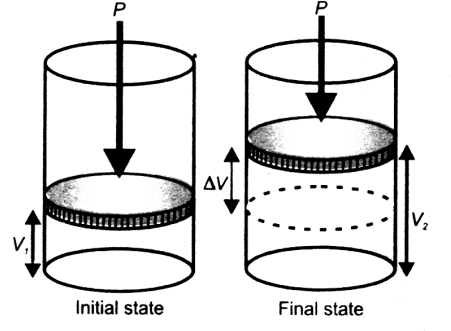
Q. what is Work?
In chemistry usually pressure volume work is taken. W = P∆V (pressure volume work)
Signs of Work
- Work done on the system is represented by +ve sign
- Work done by the system is represented by –ve sign.
1ST LAW OF THERMODYNAMICS
Q. State the first law of thermodynamics.
System cannot destroy or create energy, but it can exchange energy with its surroundings in the form of heat and work.
Mathematically
∆E = q + W
Work is done by the system. (W = -P∆V)
∆E = q – P∆V
∆E = q – P (0)
∆E = qv
ENTHALPY (H)
Q Define Enthalpy
Total heat contents of the system OR
Sum of the internal energy and product of pressure and volume
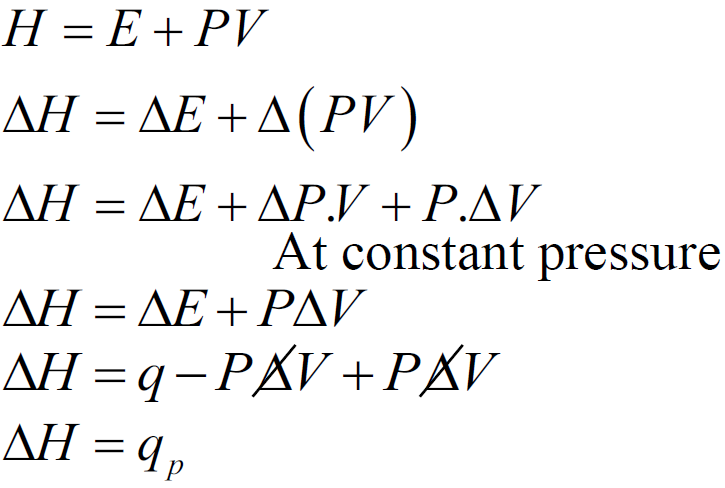
Q. Define first law of thermodynamics? Write its mathematical form?
First law of thermodynamics:
First law of thermodynamics is also called the law of conservation of energy.
OR
Energy can neither be created nor be destroyed but can be changed from one form to other.
Mathematically:
∆E = q + w
Where ∆E = change in internal energy & q = heat
w = work done
Q. Burning of candle is a spontaneous process. Justify it.
The process which can take place on its own without any external assistance is known as spontaneous process. There are some exothermic processes which need energy to start. But once reaction starts there are is no more energy required and reaction proceeds spontaneously. The burning of candle is an example of such process which needs a flame to start but once it starts burning no more energy is required.
Q. Define ENTHALPY OF REACTION (∆Hor)
Enthalpy change which occurs when certain number of moles of reactants as indicated by balanced chemical equation react together completely to give the products under standard conditions i.e. 25 °C (298K) and at one atmosphere pressure.

– 285.8 KJ mole-1 is standard enthalpy of reaction.
Q. Describe the types of enthalpies of chemical reactions.
- Standard Enthalpy of Formation (∆Hof)
Amount of heat absorbed or evolved when one mole of compound is formed from its elements under standard conditions.
Example:
- Standard Enthalpy of Atomization (ΔHoat)
Amount of heat absorbed when one mole of gaseous atoms are formed from the element under standard conditions.
Example:
- Standard Enthalpy of Neutralization (Hon)
Amount of heat evolved when one mole of H+ ions from an acid react with one mole of OH– ions from a base to form one mole of water under standard conditions.
Example: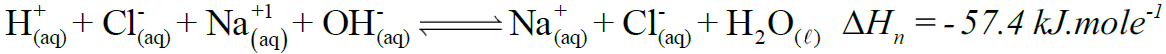
Enthalpy of neutralization of any strong acid and strong base is approximately the same i.e. -57.4 kJ mole-1. - Standard Enthalpy of Combustion (ΔHoc)
Amount of heat evolved when one mole of substance is completely burnt in excess of oxygen under standard conditions.
Example: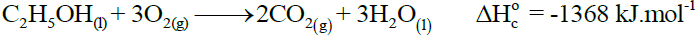
- Standard Enthalpy of Solution (ΔHos)
Amount of heat absorbed or evolved when one mole of a substance is dissolved in so much solvent that further dilution results in no detectable heat change.
Example: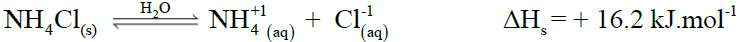
MEASUREMENT OF ENTHALPY OF A REACTION
Enthalpy of combustion and neutralization can be measured by following two methods.
1- Measure enthalpy by Glass Calorimeter.
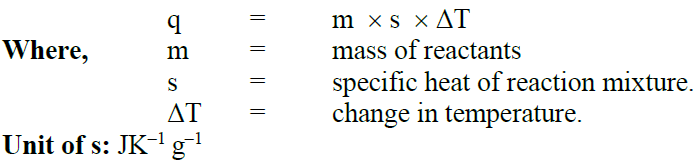
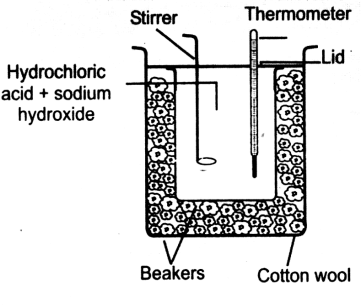
Glass calorimeter is used for the measurement of heat of neutralization and heat of solution.
2- Bomb Calorimeter:
q = c × ΔT
Unit of c: J K–1
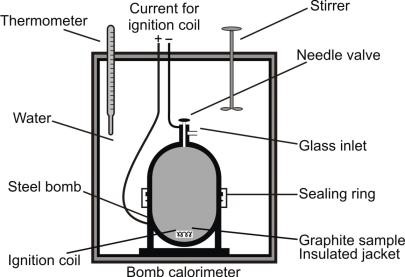
Note:
The amount of heat required to raise the temperature of one gram of substance by one Kelvin is called specific heat of the substance.
HESS’S LAW OF CONSTANT HEAT SUMMATION
Q. State the Hess’s law of constant heat summation.
Enthalpy change for a reaction by different routes is same if initial and final states are same regardless of path of reaction.
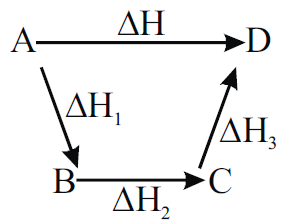
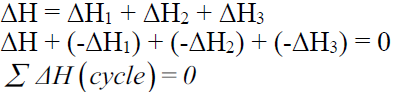
Applications:
Hess’s law is used to calculate.
Enthalpy of formation of CO, Al2O3, B2O3, CCl4.
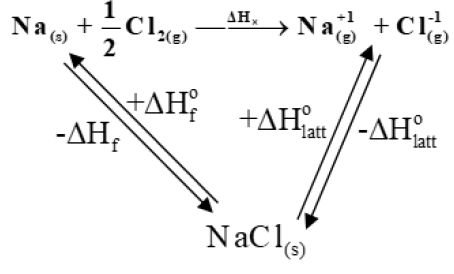
Q. Write about Born Haber Cycle.
Definition:
It states that energy change in a cyclic process is always zero.
Q. Define Lattice Energy. How is it calculated?
The enthalpy of formation of one mole of ionic compound from gaseous ions under standard conditions is called lattice energy.
Calculation of lattice energy NaCl
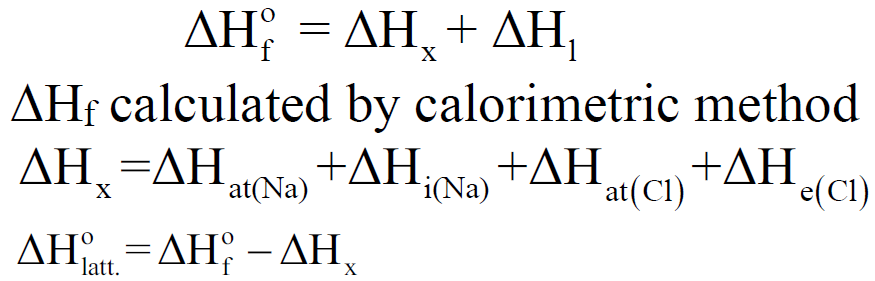
Q. Justify Hess’s law with example.
If the enthalpy of combustion for graphite to form CO2 and enthalpy of combustion of CO to form CO2 are known, then by using Hess’s law we can determine the enthalpy of formation of CO.
Consider the following cycle.
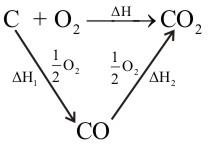
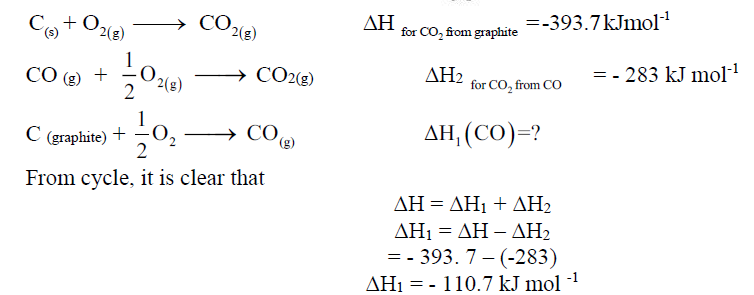
So, the enthalpy change for the formation of CO(g) is -110 kJ mol -1.
Q. What is different between heat and temperature?