Umair Khan Academy provides useful notes for classes 11 and 12. Today, reaction kinetics is under study. For other notes, quizzes, video lectures and MDCAT material visit Umair Khan Academy.
Reaction Kinetics is the last chapter in FSc Chemistry. The chapter is covered in terms of Extensive (long) and Short Questions. You can quickly revise a whole chapter in a couple of minutes.
REACTION KINETICS
Q1. Define reaction kinetics.
The study deals with the rate of chemical reactions. The rate of reaction is simply categorised as.
- Very fast rate reaction: Ionic reactions are very fast.
e.g. AgNO3(aq) + NaCl(aq) → AgCl(s) + NaNO3(aq) - Moderate rate reaction:
e.g. Hydrolysis of an ester - Very slow reactions:
1-The rusting of iron
2. The chemical weathering of rocks
Q2. Define the rate of reaction. Give an example, unit and its types.
The change in concentration of a reactant or a product per unit of time is called rate or reaction.
- Equation.
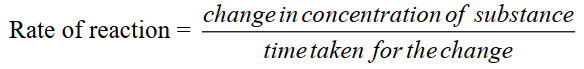
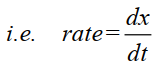
- Unit of rate of reaction: mol.dm-3. s-1.
- Instantaneous rate: Rate of chemical reaction at any instant of time.
- Average rate: Rate of reaction between two specific time intervals.
Q3. Define specific rate constant.
The rate of reaction when the concentrations of reactants are unity.
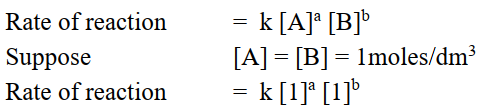
ORDER OF REACTION
Q4. Define the order of reaction. Give its types.
The sum of exponents of concentration terms in the rate equation is equal to the order of reaction.
i- 1st order reaction:
The rate depends upon the concentration of one mole of reactant.
For example. 2N2O5 → 2N2O4 + O2
ii- Pseudo 1st order reaction:
The reaction rate is independent of the initial concentration of any one of the reactants.
For example. Hydrolysis of tertiary butyl bromide.
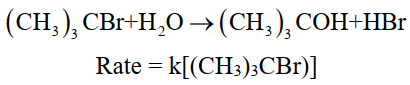
iii- 2nd order reaction:
The sum of exponents of concentration terms in the rate equation is equal to 2.
On doubling the initial concentrations of reactant the rate of reaction increases four times.
For example.
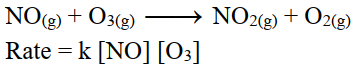
iv- 3rd order reaction:
The sum of exponents of concentration terms in the rate equation is equal to three.
On doubling the initial concentrations of reactant the rate of reaction increases eight times.
For Example.
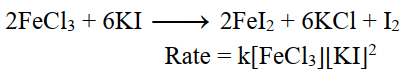
v- Fractional order reaction:
The sum of exponents of concentration terms in the rate equation is in fractions.
i.e. The reaction of chloroform with chlorine is of order 1.5.
For example.
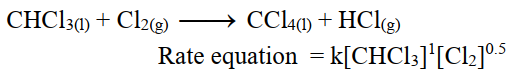
vi- Zero Order Reaction.
The rate is independent of the concentration of reactants.
For example. Photosynthesis Reaction.
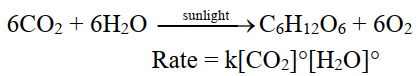
Q5. What is rate determining step?
The slowest step of a chemical reaction is called rate determining step.
Reaction Intermediate:
A species which has temporary existence during a chemical reaction is called a reaction intermediate.
Q6. Write down the methods to determine the rate of chemical reaction.
Chemical method:
It is the measurement of change in concentration of reactants or products at a regular time interval.
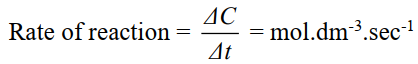
Physical Methods:
- Spectrometry:
Measurement of the amount of radiation absorbed. - Electrical conductivity method
Measurement of ions by the electrical conductivity method - Dilatometric method
Involves small volume change in the solutions. - Refractrometric method
Changes in refractive indices of the substances taking part in the chemical reactions - Optical rotation method
The angle through which plane polarized light is rotated in a polarimeter.
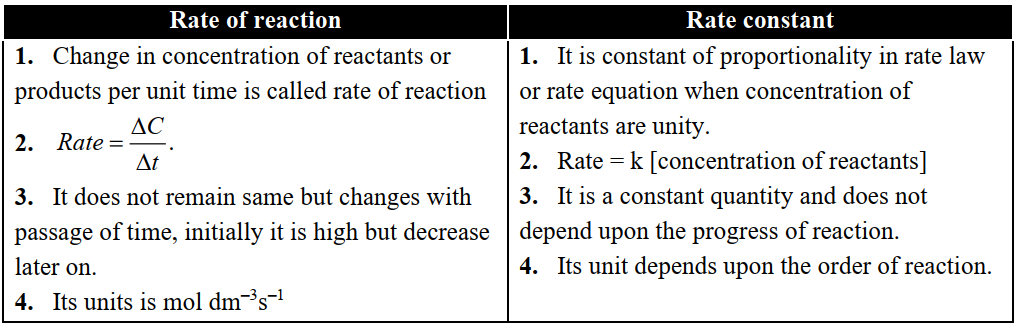
Q7. Differentiate between the rate and rate constant of a reaction.
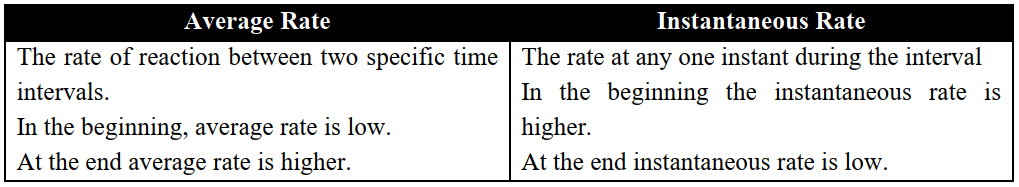
Q8. Differentiate between the Instantaneous rate and the average rate of a reaction.
Q9. What is a half-life period?
Definition: Time required to convert half of the reactants into products.
Example: The half-life period for the decomposition of N2O5 at 45oC is 24 minutes.
Relationship between half-life period and order of reaction
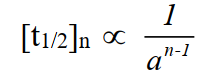
Q10. Define energy of activation.
The minimum amount of energy which is required to bring about a chemical reaction. OR
The minimum amount of energy which is required for an effective collision.
Q11. What is an effective collision?
- Effective collision:
The following conditions are required by the colliding molecules for effective collision.
- Proper orientation
- Proper energy (activation energy = Ea)
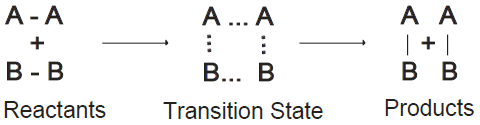
Q12. What is the Transition state?
A state in between the reactants and products. At this state, the reaction may produce a product or may go back towards the reactants. The transition state is shown in the following figure:
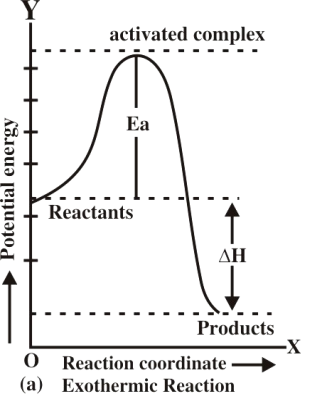
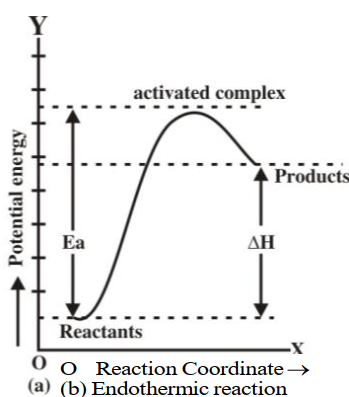
Q13. Describe Energy changes at the time of the collision graphically.
FINDING THE ORDER OF REACTION (Reaction Kinetics)
Q14. Write down the methods to find the order of reaction.
- Method of hit and trial
- Graphical method
- Differential method
- Half-life method
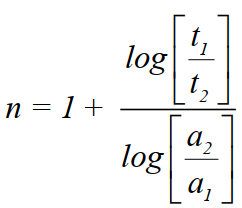
So, if we know the two initial concentrations and two half-life values we can calculate the order
of reaction (n). - Method of a large excess
One of the reactants is taken in a very small amount as compared to the rest of the reactants. The
active masses of the substances in large excess remain constant throughout.
Q15. Write down the factors affecting the rate of reaction.
- Nature of reactants
The reactivity of substances depends on the electronic arrangements in their outermost orbitals. - Concentration of reactants
The frequency with which the molecules collide depends upon their concentration. - Surface area
With the increase in surface area, the rate of reactions increases.
Example:
Powdered ‘Al’ reacts rapidly with cold NaOH because powdered ‘Al’ has a greater surface area.
2Al + 2NaOH + 6H2O → 2NaAl(OH)4 + 3H2 - Light
Each photon of light has a specific amount of energy associated with it depending on its frequency.
Example: The reaction between CH4 and Cl2 requires light. - Effect of temperature on the rate of reaction
The rate of any chemical reaction is directly proportional to the temperature.
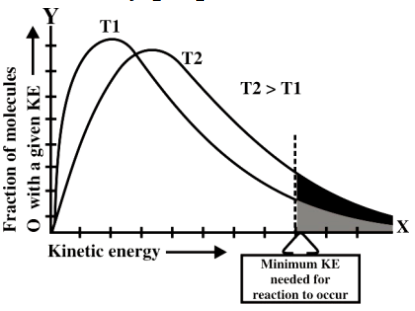
Q16. Define activation energy and activated complex. (Board Question for Reaction Kinetics)
Activation energy:
If all the collisions among reacting species at a given temperature are effective in forming the
products the reaction is completed in a very short time. Most of the reactions are slow showing
that all the collisions are not equally effective.
Activated complex:
An activated complex is an unstable combination of all the atoms involved in the reaction for which
the energy is maximum. It is a short-lived species and decomposes into the products immediately.
Since it has a transient existence, that is why it is also called a transition state.
Q17. Define the half-life period with an example. (Board Question for Reaction Kinetics)
It is mentioned earlier, the half-life of a reaction is inversely proportional to the initial concentration of reactants raised to the power one less than the order of the reaction.
Therefore, 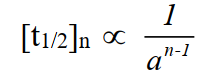
For example, the half-life of 2N2O5 → 2N2O4 + O2 is 24 minutes and the half-life of the decaying U-235 is 710 million years.
Q18. What is the Arrhenius equation?
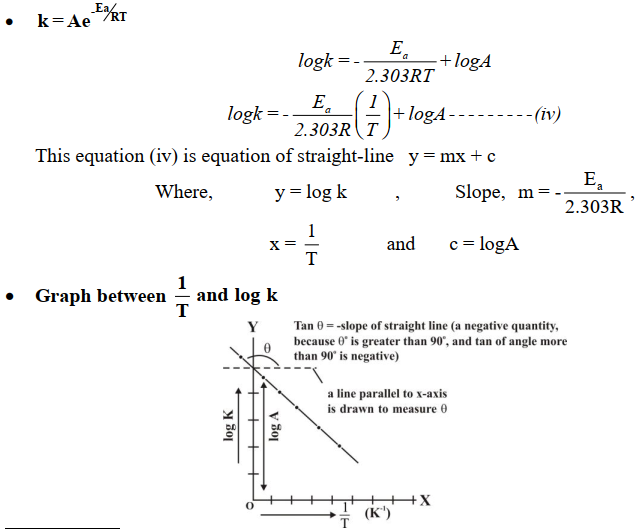
CATALYSIS (Reaction Kinetics)
Q19. Define Catalysis and catalyst and describe types of catalysts.
Catalysis.
The process takes place in the presence of a catalyst.
Catalyst.
substance which alters the rate of chemical reaction.
Types of Catalysis:
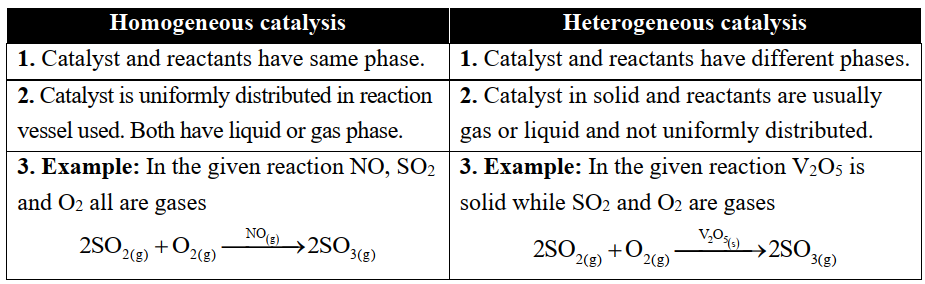
(i) Homogenous catalysis
The catalysis in which the reactants and the catalyst are in the same phase. For example;
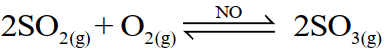
(ii) Heterogeneous catalysis
The catalysis in which reactants and the catalyst are in different phases. For example;
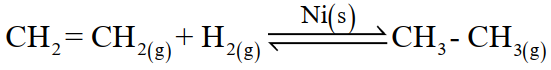
Q20. Differentiate between homogeneous and heterogeneous catalysis.
Q21. What is the poisoning of catalyst? (Board question from Reaction Kinetics)
When a catalyst becomes ineffective due to the presence of a foreign substance it is called poisoning of a catalyst. The poisoning may be temporary or permanent. Temporary poisoning can be removed and catalyst may be re-generated, a common example is the poisoning of many metallic catalysts by compounds of sulphur and arsenic. Example: As poisoning of Pt during contact process.
Q22. Write down the characteristics of catalysis.
Catalyst remains unchanged:
A catalyst remains unchanged in mass and chemical composition at the end of reaction.
Example: For the decomposition of KClO3, MnO2 is added in the form of granules.
Catalyst is required in small quantities:
Catalysts in very small amount can convert large amount of reactants into products.
Example: 1 mg of fine platinum powder can convert 2.5 dm3 of H2 and 1.25 dm3 of O2 to H2O.
Effectiveness of catalyst:
A finely divided catalyst is more effective.
Example: A lump of platinum will have much less catalytic activity than colloidal platinum.
Feasibility of a reaction and catalysts:
A catalyst cannot start a reaction which is not thermodynamically feasible.
Specificity of a catalyst:
A catalyst is specific in its action.
Example:
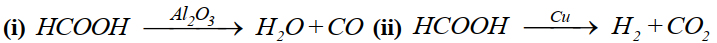
- Effect of temperature on catalyzed reactions
Temperature affects the role of a catalyst.
Example:
Colloidal catalysts like platinum may be coagulated with the rise in temperature. - Catalytic poisoning
The phenomenon in which a catalyst is made ineffective due to the presence of some foreign substances.
Q23. Write about the Activation of the catalyst.
Definition:
The phenomenon in which the activity of catalysts is increased by adding other substances.
- Activator
A substance which promotes the activity of a catalyst.
Example:
Hydrogenation of vegetable oils is catalyzed by nickel. The catalytic activity of nickel can be
increased by using copper and tellurium.
Q24. Write about negative catalysis.
Definition:
The phenomenon in which the rate of reaction is retarded by adding a substance.
Definition of inhibitor:
The substances which retard the rate of reaction.
Example:
Tetraethyl lead is added to petrol because it saves the petrol from pre-ignition.
Q25. Write about Autocatalysis.
Definition:
The reaction in which a product is formed acts as a catalyst.
Autocatalyst:
The product is formed in a reaction that acts as a catalyst.
Example:
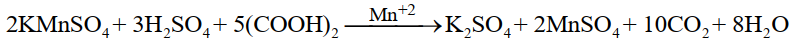
MnSO4 produced in the reaction acts as an auto-catalyst.
Q26. Write a few lines on enzyme catalysis.
Definition:
Catalysis in which enzymes act as catalysts.
Example:
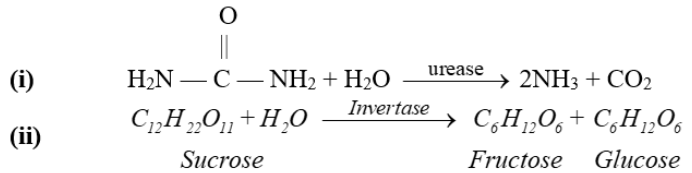
- Mechanism of enzyme catalysis
The enzymes have active centres on their surfaces. The molecules of substrate fit into their
cavities just as key fits into a lock as shown in the following figure.
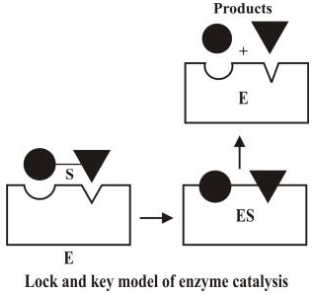
- Characteristics of enzyme catalysis
- Enzymes are the most efficient catalysts known.
- Enzyme catalysis is highly specific.
- Enzyme catalytic reactions have the maximum rates at an optimum temperature.
- The pH of the system also controls the rates of the enzyme-catalysed reaction
- The activity of the enzyme catalyst is inhibited by a poison.
- The catalytic activity of enzymes is greatly enhanced by the presence of a co-enzyme or activator.
Q27. Differentiate between auto catalysis and promoter. (Board question from Reaction Kinetics)
Auto catalysis:
In some reactions, the product produced as a result of the reaction starts acting as a catalyst right after
its production, such type of catalyst is known as auto catalyst e.g. the reaction of oxalic acid and
KMnO4 is slow at the beginning, but after some time it becomes faster because the product
MnSO4 start acting as a catalyst.![]()
Promoter:
The substance which enhances the activity of a catalyst is called a promoter. It is also known as
a catalyst for the catalyst e.g. In Haber’s process Fe is used as a catalyst but Al2O3 and Cr2O3
act as promoters.
GET IN TOUCH
Visit YouTube Channel