Umair Khan Academy provides valuable notes for the students. Electrochemistry is today’s topic. For other notes, quizzes and help visit MDCAT Preparations, Class 11 and Class 12 materials.
Following are the important short and extensive questions for electrochemistry.
INTRODUCTION TO ELECTROCHEMISTRY
Q1. Define Electrochemistry
The branch of chemistry deals with the study of the inter-conversion of electrical energy and chemical energy in electrochemical cells.
Q2. Write down the types of electrochemical cells.
- Electrolytic cell
A cell in which a non-spontaneous redox reaction takes place at the expense of electrical energy
Example: Down’s cell - Galvanic or voltaic cell
A cell in which spontaneous redox reaction proceeds to produce electricity
Example: Nickel Cadmium cell.
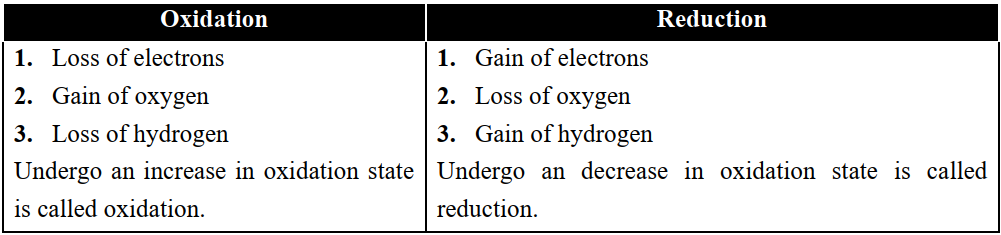
Q3. Define oxidation and reduction reactions in electrochemistry.
- Oxidation Reaction
The reactions in which substances lose electrons or gain oxygen or lose hydrogen or undergo an increase in
oxidation state are called oxidation reactions. - Reduction Reaction
The reactions in which substances gain electrons or lose oxygen or gain hydrogen or undergo a decrease in oxidation state are called reduction reactions.
OXIDATION STATE AND BALANCE THE REDOX EQUATIONS
Q4. Define oxidation State.
The apparent charge on an atom of an element in a molecule or in an ion
Example: In H+1Cl-1 oxidation state of hydrogen is +1 and that of Chlorine is -1.
Q5. Write down the rules for assigning an Oxidation Number.
- The oxidation number of all elements which are in a free state is zero.
- The oxidation number of an ion consisting of a single element is the same as the charge on the
ion. - The oxidation number of hydrogen in all its compounds is +1 except for metal hydride. It is -1 in
MH. - The oxidation number of oxygen in all its compounds is -2 except in peroxides. -1 in
peroxide, -1/2 in superoxides, +2 in OF2 - In neutral molecules, the algebraic sum of the oxidation numbers of all the elements is zero.
- In ions, the algebraic sum of the oxidation number is equal to the charge on the ion.
Q6. How to calculate the oxidation number of an element in a compound.
For example to Calculate the oxidation number (O.N) of manganese in KMnO4.
Solution:
| (Oxidation number of K) + (oxidation number of Mn) + 4(O.N of O) | = 0 |
| 1 + Oxidation number of Mn + 4 (-2) | = 0 |
| Oxidation number of Mn + (+1 – 8) | = 0 |
| Oxidation number of Mn | = +8 – 1 |
| Oxidation number of Mn | = +7 |
Thus, the oxidation state of Mn is +7 in KMnO4.
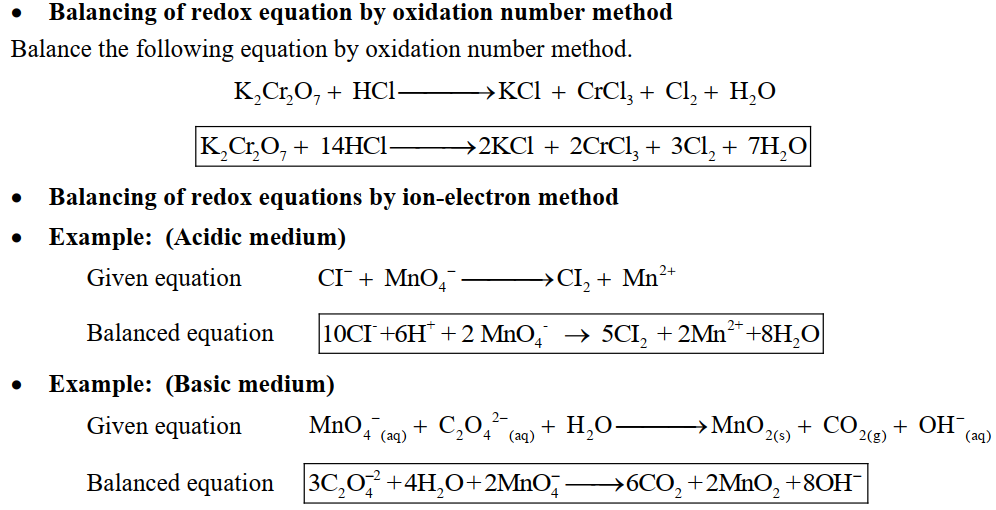
Q.7 Balance the following Equations by oxidation number Method and Ion-electron Methods correspondingly.
Q8. Differentiate between electrolytic cell and voltaic cell.
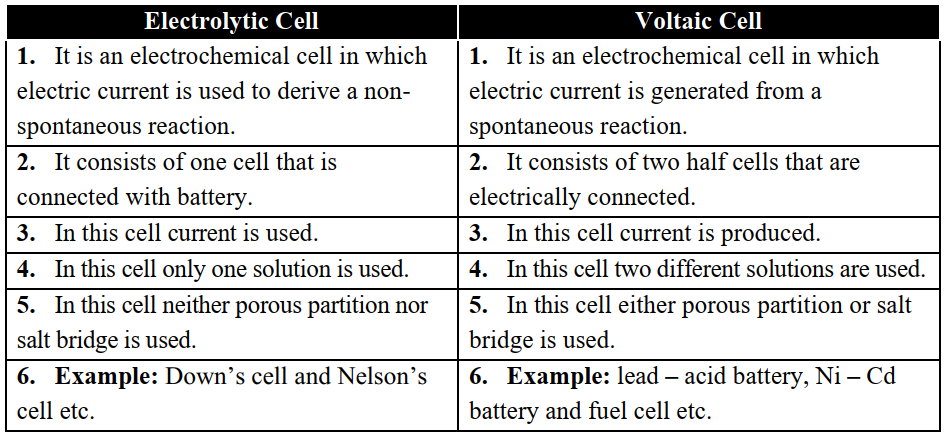
Conduction In Electrochemistry
Q9. Define Electrolytic Conduction.
The conduction in which current is carried by positively and negatively charged ions in a solution or in a fused state.
Q10. Define Electronic or Metallic Conduction.
The conduction of electricity is due to the free movement of electrons in the metallic lattice.
Q11. Define Ionization in electrochemistry.
The process of splitting of ionic compounds into charged particles when fused or dissolved in water.
Q12. Define Electrolysis.
The process in which non-spontaneous reaction takes place at the expense of electricity.
ELECTROCHEMICAL CELL
Q13. Define Electrochemical cell
A cell or a system which consists of electrodes dipped into an electrolyte in which a redox the chemical reaction uses or generates an electric current
Q14. Define electrolytic Cell.
The cell in which non-spontaneous redox reaction takes place at the expense of electrical energy
Example:
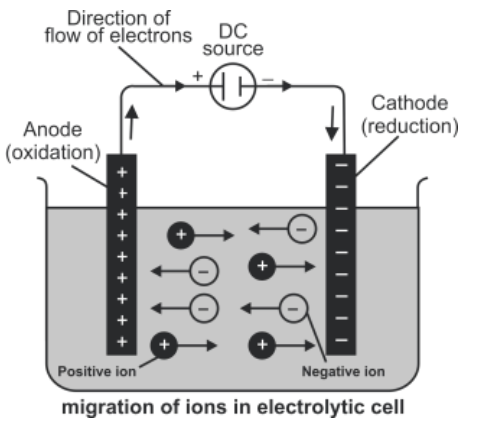
Down’s cell and Nelson’s cell are examples of electrolytic cells.
Explanation of Electrolysis
(i) Electrolysis of fused salts
Example: Electrolysis of fused lead chloride
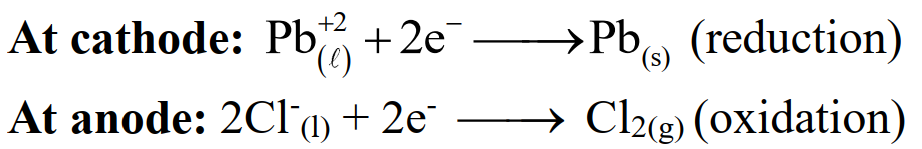
(ii) Electrolysis of aqueous solutions of salts
Example: Electrolysis of concentrated aqueous solution of sodium chloride
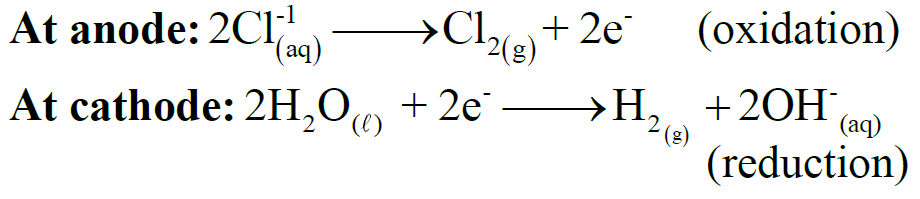
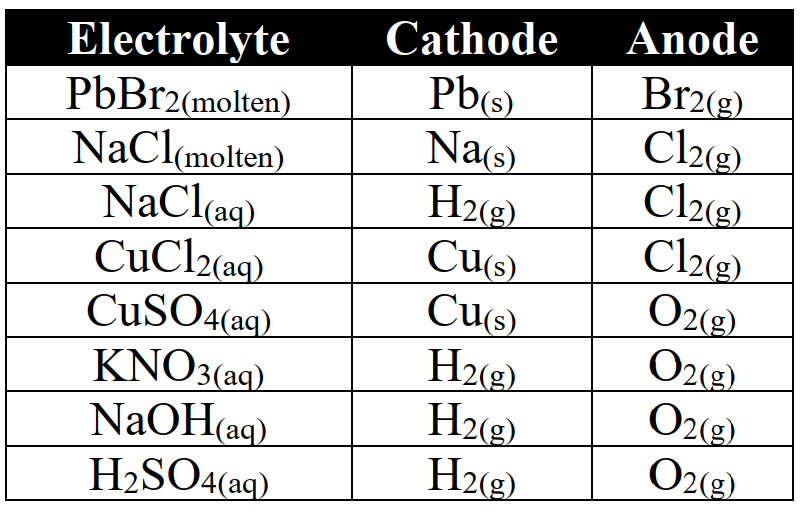
Q15. Write down the industrial importance of electrolysis.
- Extraction of sodium by Down’s Cell
- Preparation of caustic soda by Hg-cell
- Extraction of magnesium and calcium
- Extraction of aluminium
- Preparation of anodized aluminium
- Purification of copper
- Electroplating
Q15. Define Voltaic or Galvanic Cells.
The cell in which spontaneous redox reaction proceeds to produce electricity
Example: Daniel cell (Zn-Cu cell)
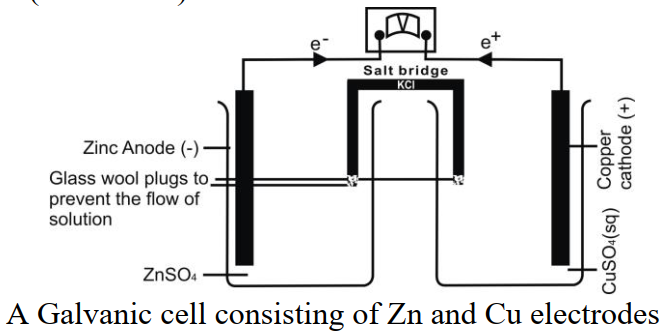
Q16. What is a Salt bridge in Electrochemistry? Write its functions.
salt bridge is a U-shaped glass tube filled with an electrolyte such as KCl in gelatin. A salt bridge serves three purposes:
- It allows electrical contact between two solutions of each half-cell.
- It prevents the mixing of the solutions.
- It maintains electrical neutrality in each half-cell.
- It prevents charge accumulation in any half of the voltaic or galvanic cell
Q17. How can copper be purified electrolytically?
Electrolytic cell can also be used for the purification of copper. Impure copper is made the anode and a thin sheet of pure copper is made the cathode. Copper sulphate solution is used as an electrolyte. The atoms of Cu from the impure Cu anode are converted to Cu+2 ions and migrate to the cathode made up of pure Cu. In this way, Cu anode is purified.
ELECTRODE POTENTIAL
Q18. Define electrode potential from an Electrochemistry Point of view.
The potential setup when an electrode is in contact with one molar solution of its own ions at 298K is called electrode potential.
Equilibrium can be represented as
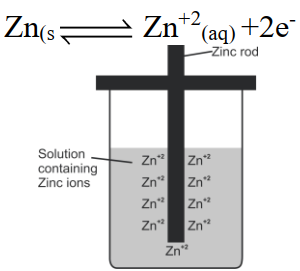
Equilibrium between zinc and its ions in solution.
Q19. Define Standard Hydrogen Electrode.
It consists of a piece of platinum foil, which is coated electrolytically with finely divided platinum black, to give it a large surface area and suspended in one molar solution of HCl. Pure hydrogen gas at one-atmosphere pressure is continuously bubbled into 1M HCl solution. The platinum acts as an electrical conductor and also facilitates the attainment of equilibrium between the gas and its ions in solution. The potential of this electrode is arbitrarily taken as zero. It is used as a standard.
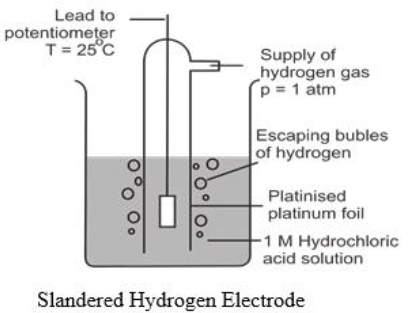
Q20. How to measure the electrode potential of any electrode.
The electrode potential of any electrode (e.g. Copper or Zinc) can be measured by making a cell of it with SHE as shown in the following figure.
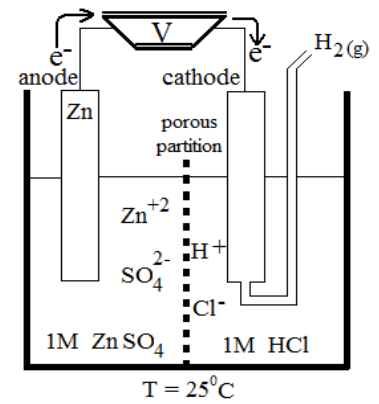
THE ELECTROCHEMICAL SERIES
Q21. Define Electrochemical Series.
The series in which elements are arranged in order of their increasing standard reduction potential (on hydrogen scale)
The standard reduction potentials (Eo) of some substances at 298K are given in the table.
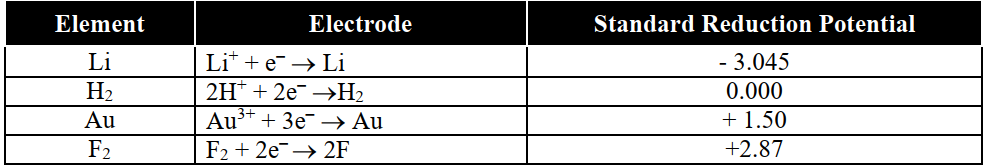
Q22. Write down Applications of electrochemical series.
- Prediction of the feasibility of a chemical reaction.
- Calculation of electromotive force (emf) of cell
- Comparison of relative tendency of metals and non-metals to get oxidized or reduced.
- Relative chemical reactivity of metals.
- Fraction of metals with dilute acids
- Displacement of one metal by another metal from its solution.
Q23. What is the spontaneity of oxidation-reduction reactions?
The spontaneity of the reaction depends upon the difference in electrode potential of the two electrodes. The greater will be the difference (E°cell), the more spontaneous will be the redox reaction. For a spontaneous redox reaction, cell voltage (E°cell) should be positive. For example in copper – zinc reaction, copper has greater reduction potential than zinc.
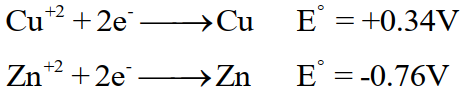
E°cell for this reaction is positive and the reaction is feasible and spontaneous.
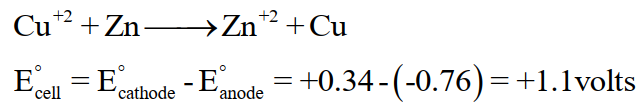
The reverse of the above reaction is non-spontaneous
MODERN BATTERIES
Q24. Define battery. What are its types.
The electric cell in which chemical change is used to produce electric current is called a battery or simply a cell.
Types of cells:
- Primary Cells:
The cells which are not rechargeable
Example: Alkaline battery - Secondary Cells:
The cells which are rechargeable
Example: Lead accumulator.
Q25. What are important Chemical reactions taking place in Lead Accumulator battery?
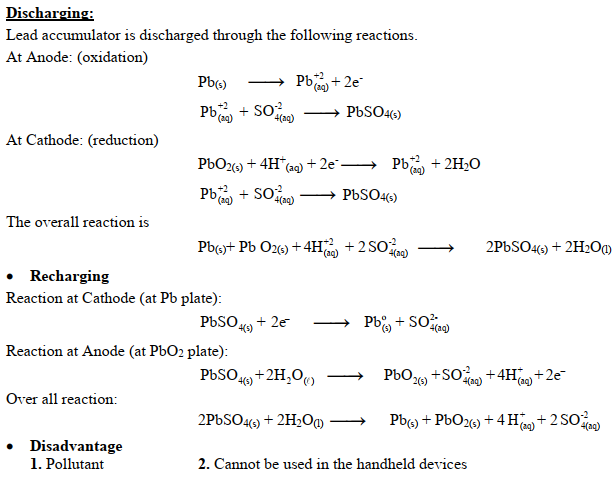
Uses:
Use in motor vehicles
Q26. Write a brief information about important batteries.
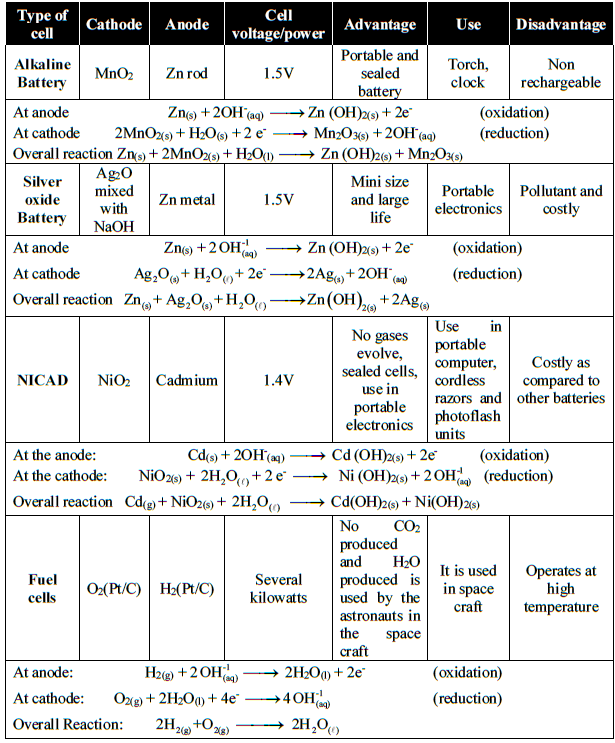
Q27. Write down the electrode reactions of the silver oxide battery.
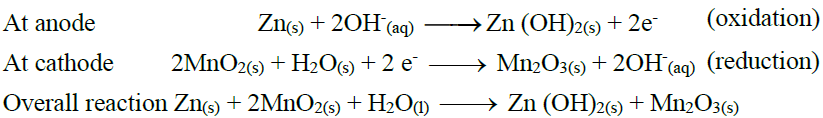
Q28. Describe Nickle Cadmium cell.
Construction:
In this cell, cadmium acts as an anode where oxidation takes place, while NiO2 acts as a cathode where reduction takes place. The electrolyte used is an alkaline solution.
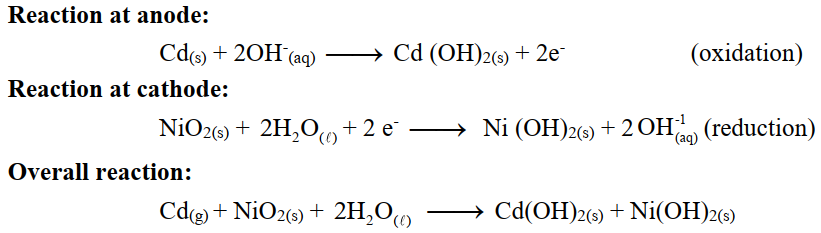
Products obtained:
The solid products which are obtained adhere to the electrodes. For this reason, the reactions are
easily reversed during recharging. Because no gases are produced during either recharging or
discharging, the battery can be sealed.
Voltage: The voltage of the cell is 1.4V. Its weight is very light.
Uses: It is used in battery-operated tools and portable computers. It also finds application in
cordless razors and photo flash units.
GET IN TOUCH
Visit YouTube Channel