Umair Khan Academy provides very useful notes for Class 11 & 12 students. Today, Class 11 Chapter 9 is going to be discussed. The chapter is named Solutions. Important short questions and extensive questions are in focus. For other notes, quizzes, video lectures and guidance visit Umair Khan Academy.
SOLUTIONS
Q1. Define solution.
Definition:
A uniform and homogenous mixture of two or more components is a solution.
Components:
- Solute: Component in small quantity
- Solvent: component in larger quantity
CONCENTRATION UNITS OF SOLUTIONS
Q2. What are the basic components of the ‘Concentration of solutions?
1-Percentage composition:
i. Percentage Weight / Weight
Weight of a solute dissolved per 100 parts by weight of solution.
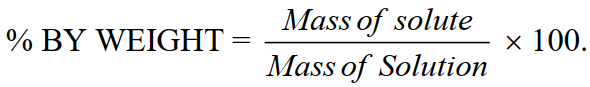
ii. Percentage Volume / Weight
Parts of solute by volume dissolved in 100 parts of solution by weight.
iii. Percentage Volume / Volume
Solute by volume dissolved in 100 parts of solution by volume
iv. Percentage Weight / Volume
Solute by weight dissolved in 100 parts of solution by volume.
2-Molarity (M)
Moles of solute dissolved per dm3 of the solution.
Unit: moldm–3 or molar.
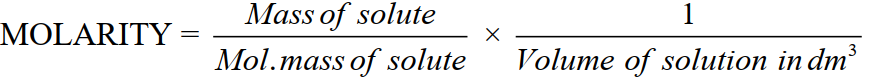
3- Molality (m)
Moles of solute dissolved in 1000g (1kg) of the solvent
Unit: mol kg–1 or molal.
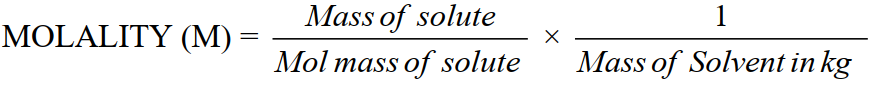
4-Mole Fraction (X)
The ratio of the number of moles of that component to the total number of moles of all the components.
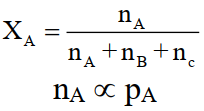
For a gas ‘No. of moles’ is directly proportional to the partial pressure of the gas. Therefore,
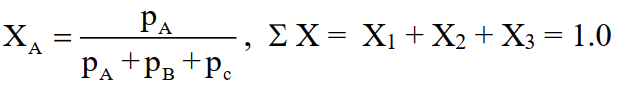
5-Mole Percent
Mole fraction multiplied by 100
6-Parts Per Million (ppm)
Number of parts (by weight or volume) of a solute present per million parts (by weight or volume)
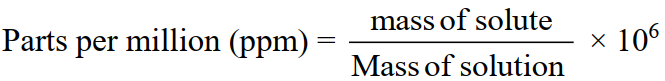
Q3. Write some interconversions of various units of concentration.
Q4. The sum of mole fractions of all the components is always equal to unity for any solution. Justify.
The sum of mole fractions of all the components will always be equal to unity because the mole fraction of any component in the solution is actually the ratio of the number of moles of it to the total number of moles of all the components present in the solution. e.g. a solution consisting of three components A, B and C having the number of moles 1, 2 and 7 then their mole fractions XA,
XB and XC will be
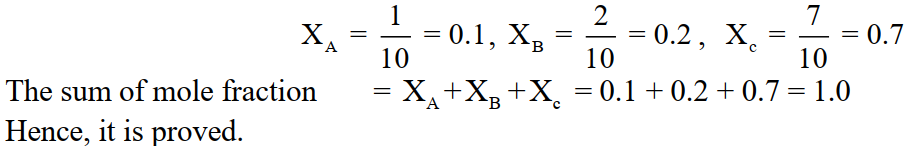
Q5. Why molality is independent of temperature but molarity depends upon temperature?
TYPES OF SOLUTIONS
Q5. What are the types of solutions on the basis of the nature of the solute and solvent?
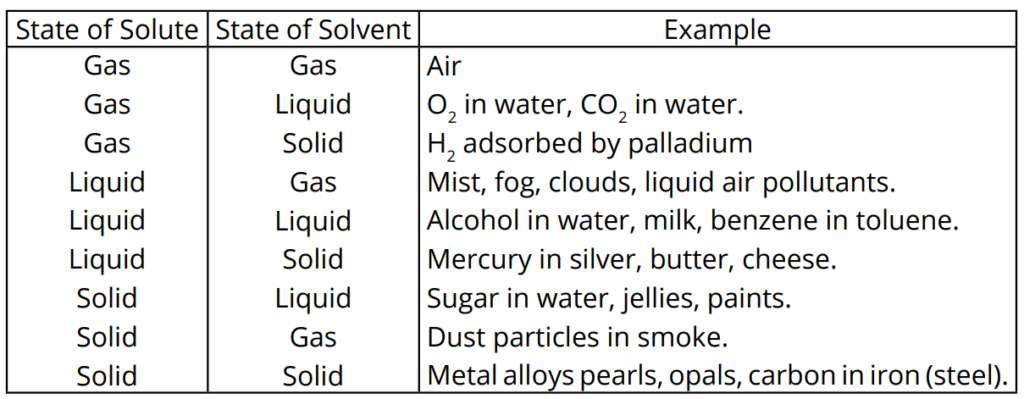
Q6. Write down the types of solutions on the basis of miscibility.
Solutions of Liquids in Liquids are divided into two types on the basis of miscibility.
- Completely miscible liquids.
(form a true solution) Mix up into all proportions
Example: Water and alcohol - Partially miscible liquids.
(form conjugated or true solution) Dissolve up to a limited extent.
Example: Ether (C2H5 —O—C2H5) dissolves water to the extent of 1.2% and water dissolves ether up to the extent of about 6.5%. - Liquids are practically immiscible. (cannot form a solution) Do not dissolve
Example: Water and Benzene (H2O + C6H6)
Q7. Define conjugate solutions.
Two partially miscible liquids form separate layers which are solutions of each other
Example: Phenol-water system.
Q8. Define Critical solution temperature or upper consulate temperature.
Temperature at which two conjugate solutions merge into each other.
For example, The solution of water and aniline has an upper consulate temperature of 167 ∘C with 15% water. While for Methanol + Cyclohexane solution it is 49.1 oC with 29% methanol.
IDEAL AND NON-IDEAL SOLUTIONS
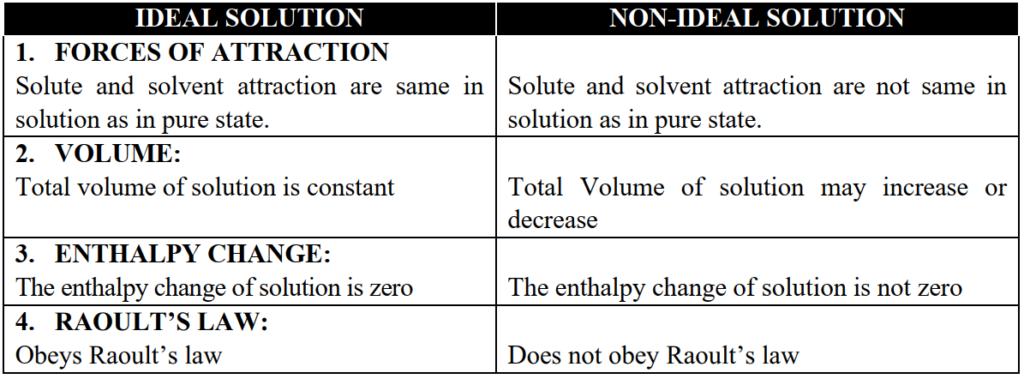
Q9. Define ideal and non-ideal solutions.
Q10. Give three statements of RAOULT’S LAW.
When only one component is volatile.
1st Statement:
The vapour pressure of a solvent above a solution is equal to the product of the vapour pressure
of pure solvent and the mole fraction of solvent in solution.
P = P∘ X1 ………….(1)
2nd Statement:
The lowering of vapour is directly proportional to the mole fraction of solute.
ΔP = P∘ X2 ……….. (2)
3rd Statement:
Relative lowering of vapour pressure is equal to the mole fraction of solute.
Relative lowering of vapour pressure.
∆P/P∘ = X2 ……….. (3)
∆P/P∘ is called the relative lowering of vapour pressure.
Q11. Write down the factors affecting the relative lowering of vapour pressure.
- Independent of the temperature.
- Depends upon the concentration of solute.
- Constant when equimolal proportions of different solutes are dissolved in the same mass of
same solvent.
Q12. Write Roult’s Law (When both components are volatile)
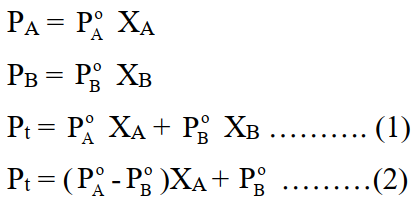
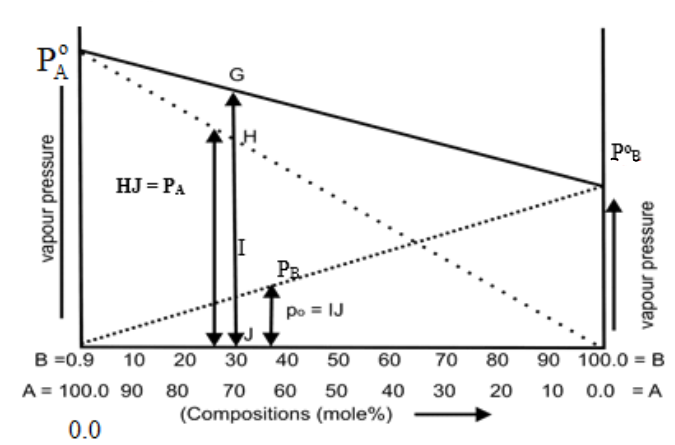
Q13. The total volume of the solution by mixing 100cm3 of water with 100cm3 of alcohol may not be equal to 200cm3. Justify it.
When 100 cm3 of water and 100 cm3 of alcohol are mixed together, the volume of solution slightly increases from 200 cm3. This mixture is non-ideal. The force of attraction among water-water or alcohol-alcohol molecules in a pure state is greater than the force of attraction between water and alcohol. This mixture deviates positively from Raoult’s law and has higher vapour pressure than the ideal pressure.
Q14. Write two points of differences between ideal and non-ideal solutions.
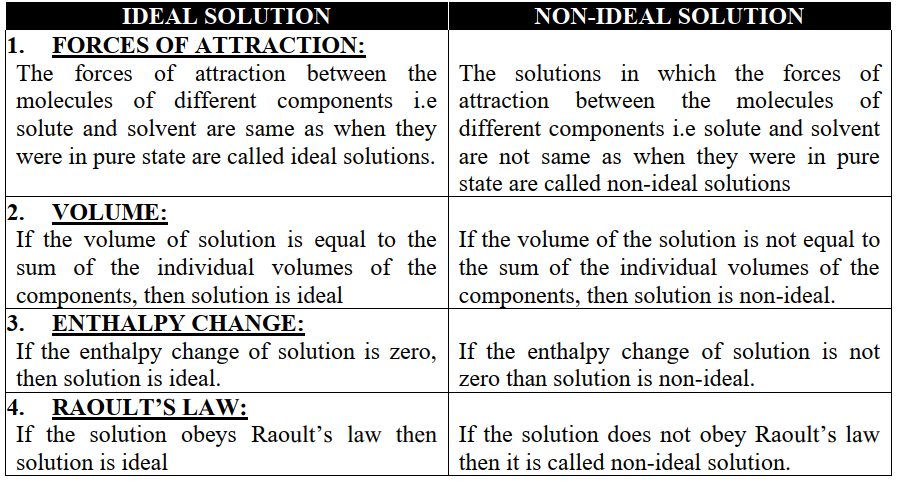
VAPOUR PRESSURE OF LIQUID-LIQUID SOLUTIONS
Q15. Write down the characteristics of ideal and non-ideal mixtures while doing fractional distillation.
Ideal mixtures: (OBEY RAOULT’S)
- Zeotropic mixtures.
- Constant boiling point
- Components can be completely separated by fractional distillation.
- Zeotropic mixtures: distil with change in composition
- Fractional distillation: separation by boiling point difference
Non-ideal solutions (do not obey Raoult’s law)
- Azeotropic mixtures.
- No constant boiling point
- Components can not be completely separated by fractional distillation.
- Azeotropic mixtures: distil without change in composition
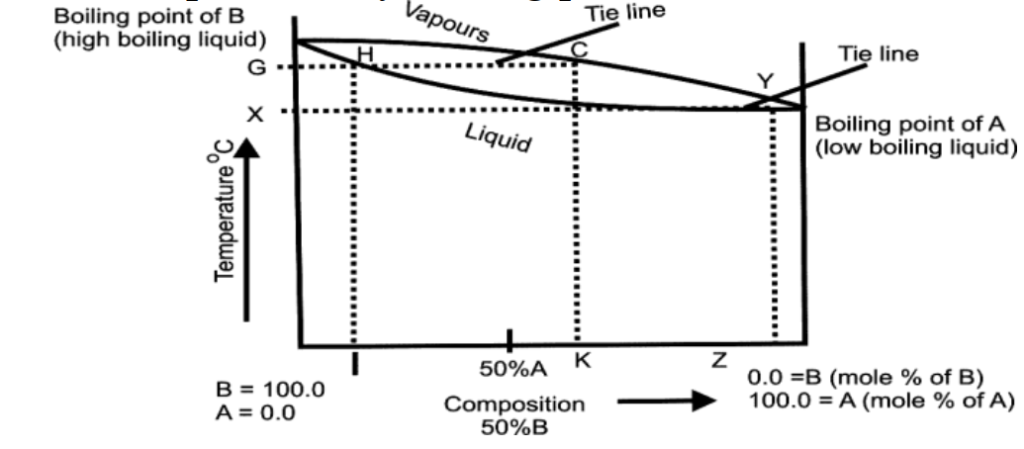
Q16. Write types of deviation of non-ideal solutions.
- a) Positive deviation:
The vapour pressure of the solution is greater than the vapour pressure of either of the pure components.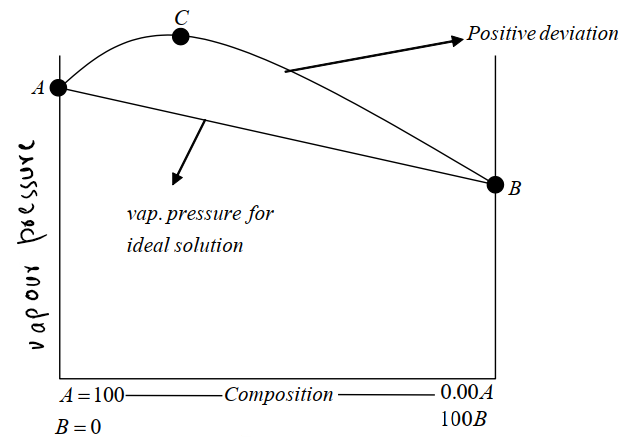
- a) Negative deviation:
The vapour pressure of the solution is lower than the vapour pressure of either of the pure components.
SOLUBILITY AND SOLUBILITY CURVES
Q17. Define solubility.
The concentration of the solute in the solution when it is in equilibrium with the solid substance at a particular temperature.
Q18. What is the effect of temperature on solubility
May increase or decrease with a change in temperature.
- The solubility of CaCl2 increases with the increase in temperature.
- The solubility of NaCl remains the same with the increase in temperature.
- The solubility of Ce2(SO4)3 decreases with the decrease in temperature.
Q19. What is a solubility cure? Give its types.
The curve drawn between the solubility and temperature is called the solubility curve.
It has the following two types.
- a) Continuous Solubility Curves:
Gradual change in solubility with change in temperature
Example: KClO3, K2Cr2O7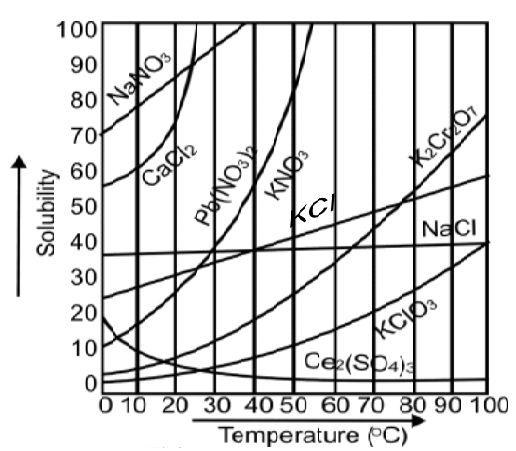
- b) Discontinuous Solubility Curves:
Sudden changes in solubilities with changes in temperature
Example: Na2SO4.10H2O and CaCl2.6H2O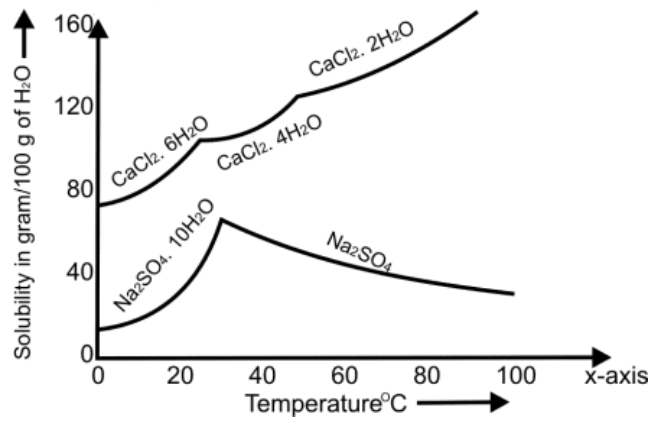
Q20. Define fractional crystallization.
Separation of solids in the form of crystals, due to differences in solubilities at different temperatures is called fractional distillation.
Q21. Define azeotropic mixture with example.
The mixtures of liquids which have constant boiling points and which distil without change in composition, i.e. components of these mixtures cannot be completely separated by fractional distillation are called azeotropic mixtures. e.g. mixture of water and ethanol.
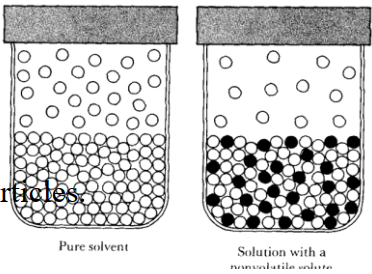
Q22. Why a non–volatile solute in a volatile solvent lowers the vapour pressure of the solution?
The particles can escape from all over the surface of pure solvent. When the solvent contains dissolved non-volatile solute particles, the escaping tendency of solvent particles from the surface of the solution decreases and its vapour pressure is lowered.
COLLIGATIVE PROPERTIES OF SOLUTIONS
Q23. Define colligative properties, and give their types.
Those properties depend on the number of solute particles and solvent molecules but not the nature of the solute.
There are four colligative properties.
- Lowering of vapour pressure.
- Elevation of boiling point.
- Depression of freezing point.
- Osmotic pressure.
These properties change their values by changing the number of solute and solvent particles.
Q24. What are the Conditions for Observing Colligative Properties?
- The solution should be diluted.
- Solute should be non-volatile
- Solute should be non-electrolyte
Q25. Write a few lines on lowering of vapour pressure.
The presence of solute particles decreases the escaping tendency of solvent particles.
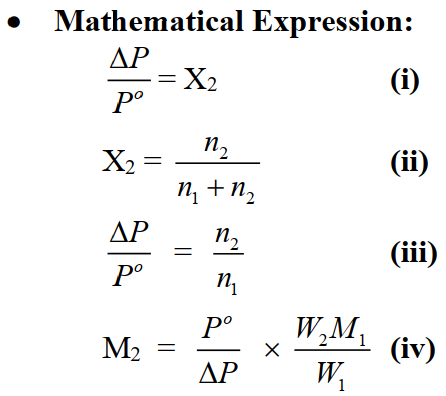
levation of the boiling point.
Definition of Boiling Point:
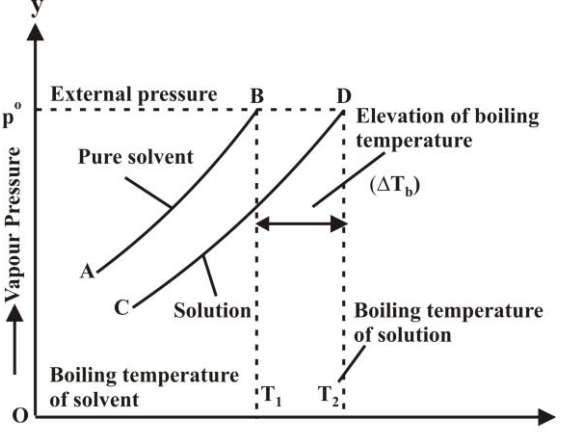
The temperature at which the vapour pressure of the liquid becomes equal to the external pressure is called the boiling point.
Elevation in Boiling Point:
Increase in boiling point of a liquid whenever a solid solute is added.
Reason for increase in boiling point:
By addition of solute, the vapour pressure decreases so the boiling point increases
Mathematically:
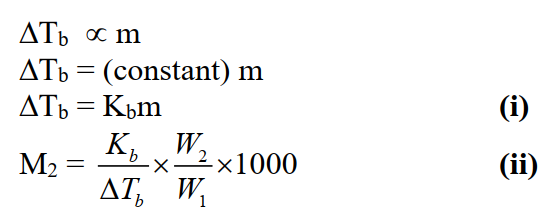
Molal boiling point constant or ebullioscopic constant (Kb):
Elevation of the boiling point of a solvent when its one molal solution is formed
Example:
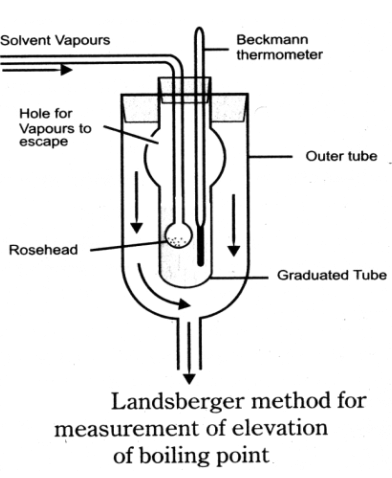
Q27. How to measure boiling point elevation: Landsberger’s Method:
This is the best method for the measurement of the elevation of boiling point.
Construction:
- The inner tube with a hole in its side. This tube in
graduated. - A boiling flask sends the solvent vapours into graduated
tube through a rose head. - An outer tube, which receives hot solvent vapours coming from
the side hole of the inner tube. - A thermometer which can read upto 0.01K.
Q28. Write a few on the depression of the freezing point.
Definition of the freezing point:
Temperature at which the solid and liquid phases of the substance co-exist
- Depression in freezing point
Decrease in freezing point of a liquid whenever a solid solute is added - Reason for decreasing in freezing point:
By the addition of solute in solvent, the vapour pressure decreases so the freezing point decreases.
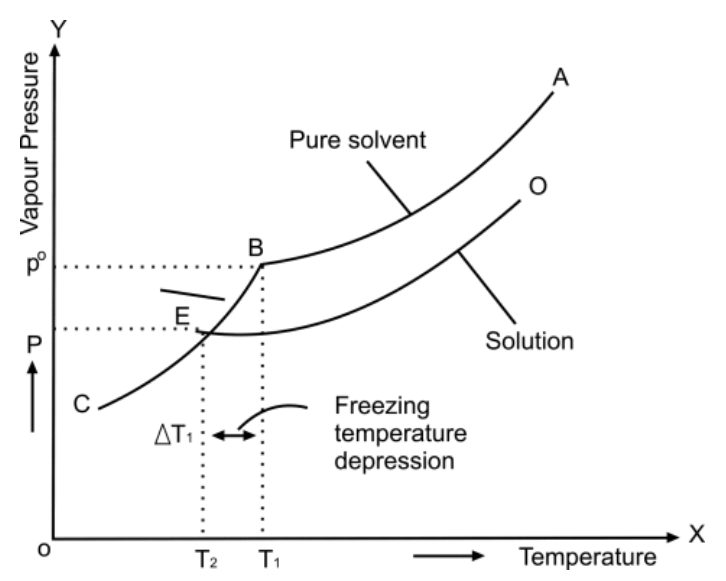
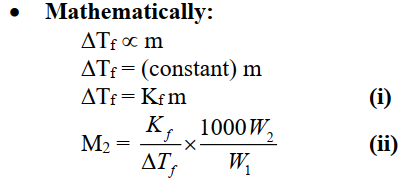
Cryoscopic constant or molal freezing point constant (Kf):
Depression in the freezing point of solvent when its one molal solution is formed
Example:

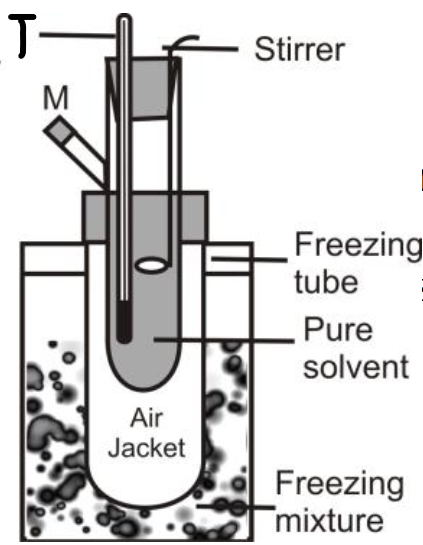
Q29. How to measure the freezing point depression by Beckmann’s method.
Beckmann’s Freezing Point Apparatus:
Beckmann’s method is easy to perform.
- Construction of the apparatus:
- A freezing tube with a side arm. It contains solvent or solution and is fitted with a stirrer and a Beckmann’s thermometer.
- An outer larger tube into which the freezing tube is adjusted. The air jacket in between these tubes helps to achieve a slower and more uniform rate of cooling.
- A large jar containing a freezing mixture.
Q30.What are the applications of boiling point elevation and freezing point depression phenomenon?
- 1. Molecular masses of non-electrolytes and non-volatile solutes can be determined.
- 2. Increases liquid range.
- 3. Use of an antifreeze solution in the radiator of an automobile. The antifreeze solution also protects the radiator from boiling over.
- 4. NaCl or KNO3 is used to lower the melting point of ice.
Q31. Why freezing point is depressed due to the presence of solute?
The freezing point is the temperature at which its solid and liquid phases have the same vapour pressures. When a solute is added to a solvent, its vapour pressure is decreased. Because of this decrease in the vapour pressure of the solvent, the freezing point is also decreased.
Q32. In summer the antifreeze solutions protect the radiators from boiling over. How?
Water is used as a coolant in the radiators to decrease the temperature of the working engine. But in summer sometimes water starts boiling over due to hot weather. If we add some suitable solutes which increase the boiling point of water above 100°C. We can easily protect the radiator from boiling over.
ENERGETIC OF SOLUTIONS
Q33. Define the energetics of the solution.
The change in energy during solution formation is called solution energetics.
Q34. Define Enthalpy or heat of solution.
The heat energy that is absorbed or released at constant pressure when a substance forms a solution is called heat of solution or enthalpy of solution.
Example:
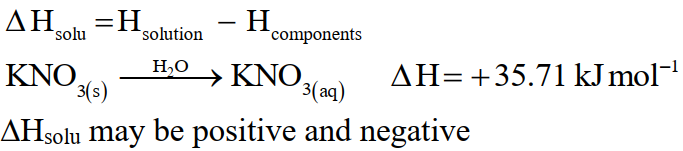
Q35. Define Hydration Energy.
The energy released when one mole of ions are hydrated (solvated) by solvent molecules
Examples.
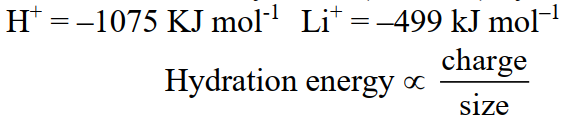
Q36. Define Lattice energy.
The energy needed to separate one-mole ions from its crystalline compound is called lattice energy. Or The amount of energy needed to break one-mole bonds of the crystal lattice to release ions is called lattice energy. This energy is related to hydration energy by the following expression.
- 1. ∆Hlatt. < ∆Hhyd. = – ∆H(dissolution) (Exothermic)
- 2. ∆Hlatt.. > ∆Hhyd. = + ∆H(dissolution) (Endothermic)
HYDRATION AND HYDROLYSIS OF SOLUTIONS
Q37. Define hydration.
Process in which water molecules surround and interact with solute ions or molecules.
Q38. Define Hydrates.
Crystalline substances which have chemically combined water molecules are called hydrtes.
Formation of hydrates:
Hydrates are mostly formed when aqueous solution of soluble salt is evaporated.
Example:
- (COOH)2.2H2O (Oxalic acid)
- MgSO4.7H2O (Epsom salt)
Q39. What is the water of crystallization:
Water molecules which become part of crystals during the process of crystallization are called water of crystallization.
Q40. Define hydrolysis and explain it.
the reaction of the anion or cation of a salt with water is called hydrolysis.
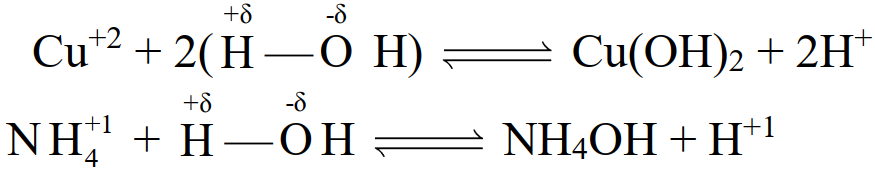
- Salts of Strong Acids and Strong bases:
Salts of strong acids and strong bases such as NaCl. Such salts are dissociated into ions but don’t
react with water and form a neutral solution. - Salts of weak acids and strong bases:
Salts of weak acids and strong bases such as CH3COONa, such salts hydrolyse water and give
basic solution.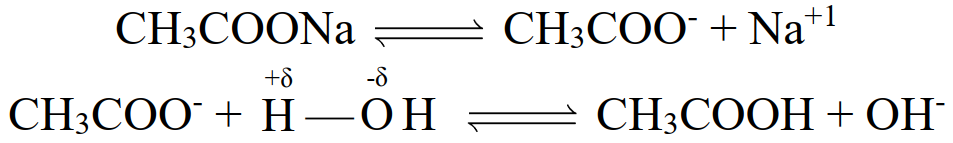
- Salts of Strong Acid and Weak Bases:
Such as NH4Cl, hydrolyse water and give an acidic solution.
- Salts of weak acid and weak bases:
Salts of weak acids and weak bases such as CH3COONH4 are dissociated in water to a small extent. It is not necessary that solutions of these salts are neutral.
If pKa = pKb mean that the solution is neutral.
If pKa > pKb then the solution is basic.
If pKa < pKb then the solution is acidic.
Q41. Explain why CuSO4 give an acidic solution when put in H2O.
Copper sulphate when dissolved in water. It reacts with water and produces an acid-base
pair through the following reaction.
CuSO4 + 2H2O → Cu(OH)2 + H2SO4
Copper hydroxide is a weak base whereas sulphuric acid is a strong acid so overall the solution
will be acidic in nature.
Q42. Why the hydration energy of Na+ is less than the Li+ ion?
Hydration energy is directly related to the charge density of an ion smaller the size, the greater the charge density and vice versa. That’s why Li+ has a smaller size, greater charge density and greater value of hydration energy than Na+.
Example: The hydration energies of Li+ and Na+ are –499 kJ/mol and –390 kJ/mol respectively.
GET IN TOUCH
Visit YouTube Channel



there is no complete notes of ch 11 not present d/f of minimum and maximum aezotopes.
For what board are you preparing. In Punjab board minimum and maximum azeotropes are not included in detail, but only a difference between zeotropes and azeotropes.