This section provides you with Class 12 Notes regarding very important Short Questions. These Questions cover almost all previous board questions from chapter 4(Group V-A and VI-A elements. Notes for other chapters are also available in class 11 and 12 sections.
Here is a video discussion for the following important short questions.
Q1. How does nitrogen differ from other elements of its group?
- Nitrogen is a gas while the other elements of the same group are solids.
- Nitrogen is diatomic while the other elements of the same group show tetra atomic Oxidation State.
- Nitrogen occurs in a free state and combined state while the other elements of the group V-A occur in a combined state only.
Q2. How does Aqua regia dissolve gold and Platinum?
When one volume of the Conc. HNO3 is mixed with 3 volumes of the Conc. HCl, aqua regia is obtained which is used to dissolve gold and platinum.
HNO3 + 3HCl → NOCl H2O + Cl2
2NOCl → 2NO + Cl2
This liberated Cl2 reacts with noble metals like Au and Pt to convert them into chlorides.
Q3. Why the elements of group VIA other than the oxygen show more than two oxidation states.
The elements of group VIA other than the oxygen show more than +2 oxidation because they have empty d orbital and due to the presence of different numbers of unpaired electrons in orbitals (ns, np, nd) of valance shell they show +2, +4 and +6 oxidation states.
Q4. Write down the comparison of the properties of oxygen and Sulphur.
| Similarities | Differences |
|---|---|
| 1. Both Oxygen and sulphur have the same outer electronic configuration of ns2 np4 2. Both Oxygen and sulphur are usually divalent. 3. Both have polyatomic molecules oxygen has diatomic, O2, while sulphur has S2 and S8 molecules 4. Both Oxygen and sulphur exhibit allotropic forms. Both are typical non-metals. 5. Both are found in free and combined States on earth | 1. Oxygen is a gas while sulphur is solid at ordinary temperature. 2. Oxygen is sparingly soluble in water while sulphur is not soluble in water. 3. Oxygen helps in combustion while sulphur is itself combustible. 4. Oxygen is paramagnetic in nature while sulphur is diamagnetic in nature. 5. Oxygen does not react with acids while sulphur is readily oxidised by concentrated H2SO4 or HNO3. |
Q5. Write down the equation for the reaction between the concentrated H2SO4 and copper and explain what type of reaction is it.
Cu0 + 2H2S+6O4 → Cu+2SO4 + 2H20 + S+4O2
In this reaction, copper is oxidized and sulphur is reduced, which is called an oxidation-reduction reaction.
Q6. Explain the Brikeland and Eyde’s process for the manufacturing of the HNO3.
The following steps are involved.
- Formation of nitric oxide
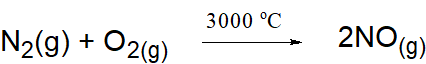
NO formed is cooled quickly to 2000°C at which it does not decompose - Oxidation of nitric oxide
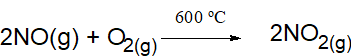
- Dissolution in water

- Oxidation of nitrous acid
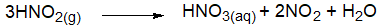
Q7. Which metal evolve hydrogen up on reaction with HNO3? Illustrate along with the chemical equations.
Magnesium, calcium and manganese give hydrogen with dilute HNO3
![]()
![]()
Q8. What is meant by fuming HNO3?
A mixture of concentrated HNO3 and NO2 is called fuming HNO3 it is more reactive than concentrated HNO3 it is a red or pale yellow fuming liquid.
Q9. H2SO4 is said to act as an acid, an oxidizing agent and dehydrating agent, describe two reactions in each case to illustrate the truth of this statement.
H2SO4 as Acid
![]()
![]()
H2SO4 as Dehydrating Agent
![]()
H2SO4 as Oxidizing Agent
Sulphur is oxidized as![]()
![]()
Q10. Give the advantages of contact process for manufacture of H2SO4.
The following are the advantages of the contact process;
- By contact process, we can get H2SO4 of any required concentration.
- It is environmentally friendly and no by-products are formed.
- It is used in commercial preparation.
- The catalyst used is easily handled
Q11. Describe the chemistry of the industrial preparation of H2SO4 from sulphur by contact process.
Following are the major steps involved in the chemistry of H2SO4 formation.
Q12. Why is SO3 dissolve in H2SO4 and not in water?
When sulphur trioxide is dissolved in 98% H2SO4 then oleum is produced which can easily be converted into H2SO4 by dissolving in the water while SO3 is not dissolved in water directly because H2SO4 of the required concentration cannot be prepared as SO3 is less soluble in water at high temperature as well as the reaction is highly exothermic.
Q13. Explain the action of H2SO4 on metal along with the chemical equations.
Cold dilute H2SO4 react with almost all metals to produce hydrogen gas![]()
Cold-concentrated H2SO4 does not react with most metals like Cu, Ag, Hg, Pb, Au etc. But hot concentrated H2SO4 can react with these metals in this way;![]()
Q14. Describe the preparation of NO2 gas also give its reactions.
- By heating Lead Nitrate
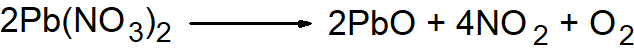
- By reacting concentrated HNO3 with copper.
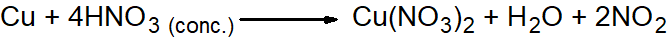
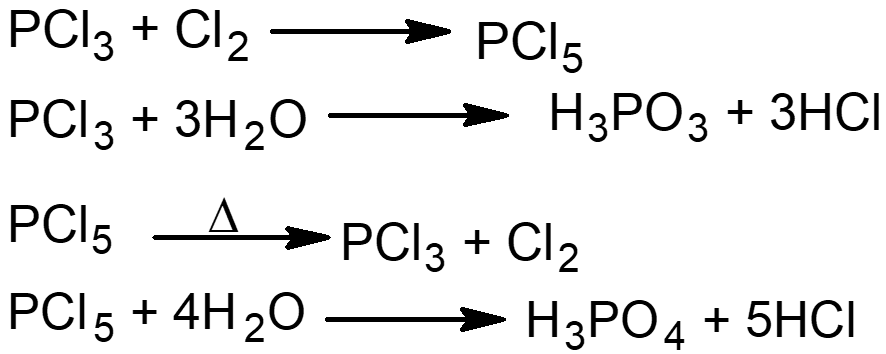
Q15. How doesPCl3 in PCl5 can be used for the preparation of other chemical compounds.
Q16. Describe ring test for the confirmation of the presence of the nitrate ion in a solution
The given Nitrate solution is dissolved in water and some fresh FeSO4 solution is added to it. Concentrated H2SO4 is slowly run down the side of the test tube, brown ring is formed at the junction of the two solution which confirms the presence of a nitrate ion in a given solution.
Q17. NO2 is a strong oxidizing agent. Prove the truth of the statement giving examples.
NO2 is strong oxidizing agent and oxidizing H2S to sulphur, ferrous sulphate to ferric sulphate and potassium iodide to Iodine. Some of examples are given below
- H2S + NO2 → H2O + S + NO
- 2KI + 2NO2 → KNO2 + I2
Q18. Write down the chemical equations and names of the products formed as a result of the reaction of HNO3 with arsenic and antimony.
Arsenic and antimony are metalloids they can be oxidized to their corresponding acids when react with concentrated HNO3.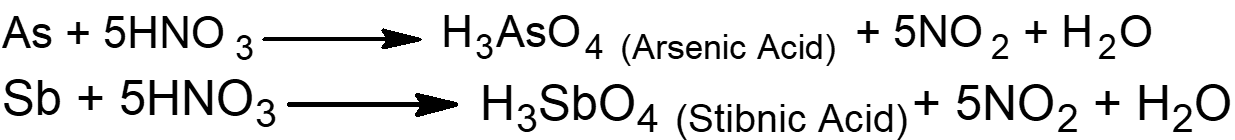
Q19. Give the methods of preparation of PCl3.
- It is usually prepared by melting white phosphorus in a retort in an inert atmosphere of carbon dioxide and the current of the dried chlorine is passed over it. The vapours of PCl3 are collected in a flask kept in the ice bath.
2P + 3Cl2 → 2PCl3 - It is also prepared by the action of phosphorus with thionyl chloride.
2P + 4SOCl2 → 2PCl3 + 2SO2 + S2Cl2
Q20. P2O5 is a powerful dehydrating agent. Prove giving example.
The following examples prove that P2O5 is a powerful dehydrating agent.![]()
![]()
![]()
Q21. Describe the methods of preparation of phosphorus pentachloride and explain its reactions.
- By passing dry chlorine to Phosphorus trichloride at a temperature of about 0°C, phosphorus pentachloride is formed.
PCl3 + Cl2 → PCl5 - It is also prepared by passing dry chlorine in a well-cool phosphorus solution in carbon disulphide.
2P + 5Cl2 → 2PCl5
Q22. Discuss the trends in physical properties of the VA elements.
- Metallic character increases down the group.
- Trend down the group, shifts from covalent bonding to ionic bonding.
- As we move down the group the oxidation states increase.
Q23. Name three allotropic forms of phosphorus.
Some of the allotrophic forms are White Phosphorus, red phosphorus, and black phosphorus.
Q24. How does HNO3 act as an oxidizing agent?
It acts as oxidising agent due to the ease with which it is decomposed.![]()
Q25. How does P2O5 reacts with water in cold and hot state?
- In a cold state metaphosphoric acid is formed
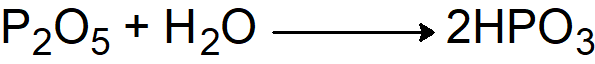 .
. - In a hot state orthophosphoric acid is formed
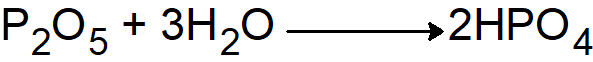
Q26. Write any four important uses of H2SO4.
- It is used to manufacture fertilizers like Ammonium Sulphate and Calcium superphosphate.
- It is used to refine petroleum to remove nitrogen and sulphur compounds.
- It is used in the manufacturing of HCl phosphoric acid HNO3 and sulphates.
- It is used in electric batteries and storage cells
Q27. Nitrous acid decolourizes acidified KMnO4 and bromine water. Give reactions.
Q28. Write down the structures of different oxides of Nitrogen.
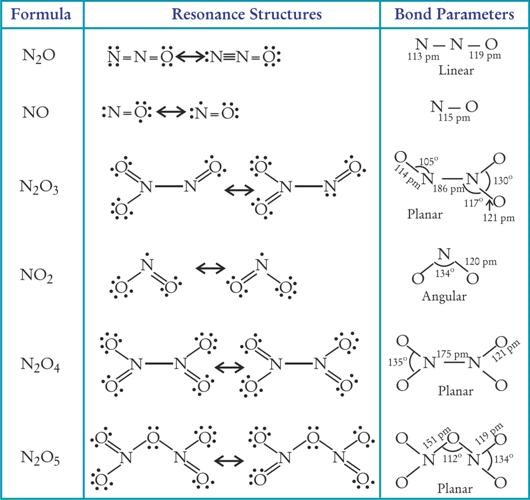
Q29. N2O supports combustion. Give two reactions in its favour.
Q30. Why dinitrogen oxide is called laughing gas.
When dinitrogen oxide is inhaled for a sufficient time it produce a laughing sensation so it is called a laughing gas.
Q31. Give name and formulas of oxyacids of phosphorus.
- Phosphoric acid (H3PO3)
- Orthophosphoric acid (H3PO4)
- Pyrophosphoric acid (H4P2O7)
- Metaphosphoric acid (HPO3)

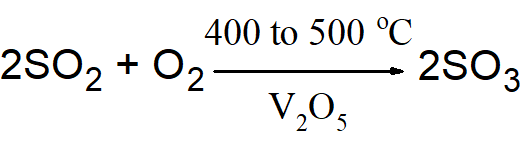


Thnkq sir