‘Transition Elements’ is the last chapter in the Inorganic section. Generally, it describes basic knowledge about Transition Elements (d-block elements) with their properties and uses. Umair Khan Academy provides necessary notes for exam preparations. As this chapter is important in exams due to the short questions, therefore, short questions are given below. For more notes and help visit Umair Khan Academy.
A video discussion is also provided here for your easy understanding for the following exams questions.
Q.1 Define the following with examples.
Ligands
The atoms, ions or molecules, which surround the central metal ion and donate electron pairs to it, are called ligands. e.g.
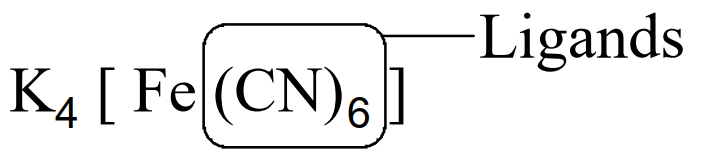
Coordination sphere
The central metal atom or ion along with the ligands is called the coordination sphere. It is placed in square brackets.
It may be anion, cation or neutral.
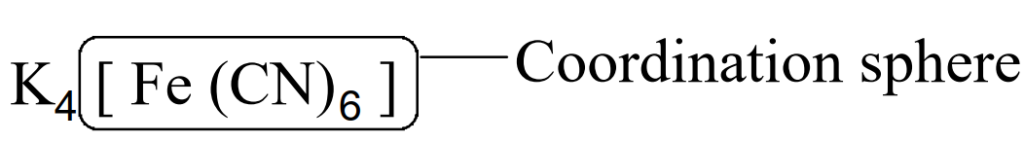
Coordination Number
The number of lone pairs of electrons provided by the ligands to the central metal atom or ion is called the coordination number of the central metal atom or ion. e.g.
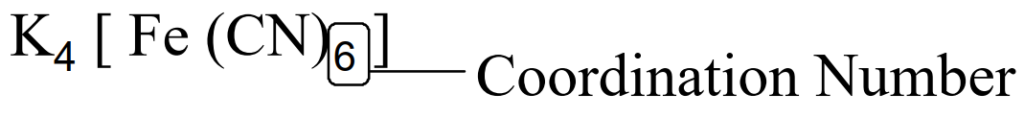
Central metal ion
A metal atom or ion is usually a transition element surrounded by a number of ligands, called a central metal atom or ion. e.g.

Charge on coordination sphere
It is the algebraic sum of the charges present on the central metal ion and the total charge on the ligands.
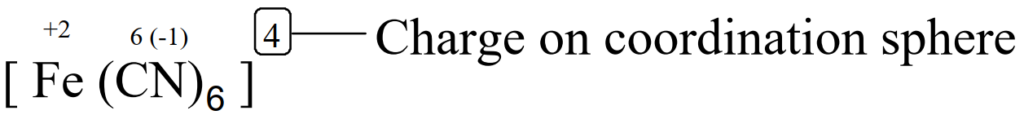
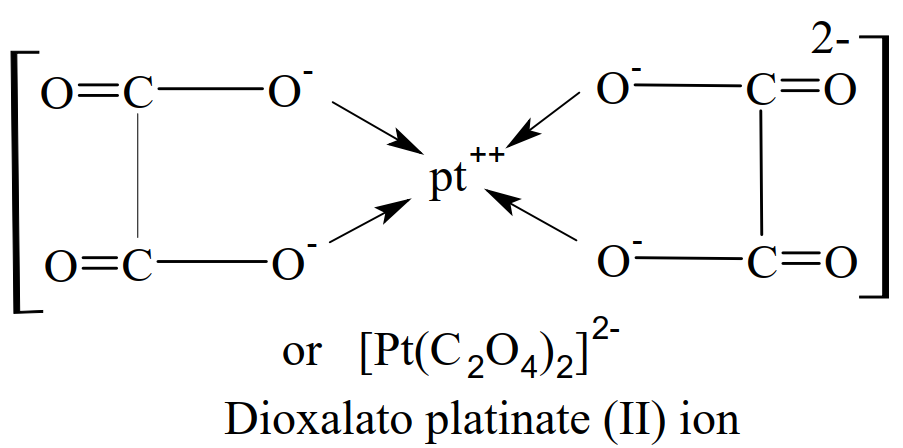
Chelates
When all the donor atoms of a polydentate ligand get coordinated with the same metal ion, a complex compound is formed which contains one or more rings in its structure, called chelate. e.g.
Q.2 Define substitutional alloy. Give an example.
Due to the similarity in sizes of transition metals, some transition metal atoms are able to replace on another in the metallic lattice and form substitutional alloy among themselves. The alloy of steel is an important example of this type of material in which iron atoms are substituted by chromium, manganese and nickel atoms, etc. to give steel more useful properties.
Q.3 What are the differences between wrought iron and steel?
Wrought iron
It is the purest form of commercial iron and contains the lowest % age of carbon and up to 0.3% of impurities like
S, P, Si and Mn etc.
Steel
Steel is the alloy of iron containing 0.25% carbon and traces of S, P, Si Mn etc.
Q.4 Why does damaged tin-plated iron get rusted quickly?
If the protecting coating of tin is damaged then the iron comes into contact with moisture. A galvanic cell is established in which tin acts as a cathode and iron acts as an anode. The electron moves from iron to tin, where it discharges H+ ions, leaving behind OH- in the solution. These OH- ions react with iron and form Fe(OH)3, which dissolves rapidly in water.
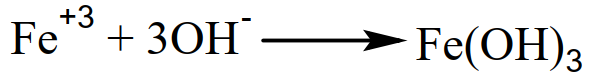
So the damaged tin-plated iron gets rusted quickly due to the fact that water promotes the electrochemical process.
Q.5 Under what conditions does aluminium corrode?
When an active metal Al comes in contact with less active metals like Zn, Cu, Fe, and Sn (having greater reduction potential than Al) a galvanic cell is established in which aluminium acts as an anode while metals like Zn, Fe etc. act as cathode. In this process, active metal (Al) corrode rapidly while the other remains intact.
On the other hand, the main condition of rapid corrosion of Al is when the protective layer is damaged and the metal comes in direct contact with water. Besides dissolving the compounds, water also allows the corrosion to penetrate further into the metal and promotes the electrochemical process.
Q.6 How the process of galvanizing does protect iron from rusting? OR
What is sacrificial corrosion?
When a clean sheet of iron is dipped in a zinc chloride bath and heated, the iron sheet is then removed, rolled into a Zn bath and air cooled. This process is called galvanizing or zinc coating.
If the sheet of the zinc is damaged a galvanic cell is established in the presence of moisture. Iron serves as a cathode and zinc acts as an anode. Electrons flow from Zn to iron, so Zn corrodes and Fe remains intact and in this way, iron is protected from rusting. This type of corrosion is called sacrificial corrosion
Q.7 How chromate ions are converted into dichromate ions.
In an aqueous solution, CrO42- (yellow colour) and Cr2O72- (orange-red) ions exist in equilibrium.
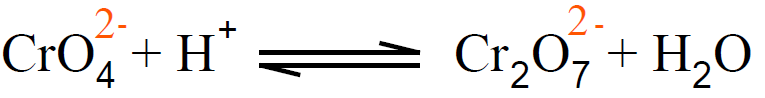
When an acid is added in such a solution then the H+ ions from the acid increase in its conc. According to Le-Chatelier’s principle, if the concentration of reactants increases at the equilibrium stage this will disturb the position and the reaction is shifted in the forward direction. So in this way, CrO42- ions are converted into dichromate ions (Cr2O72-).
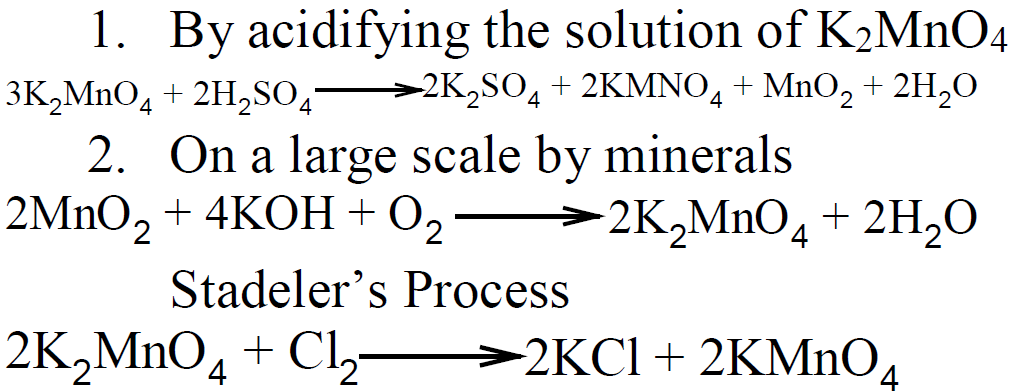
Q.8 Describe the preparation of KMnO4 and K2CrO4.
1- Preparation Of KMnO4
2- Preparation of K2CrO4
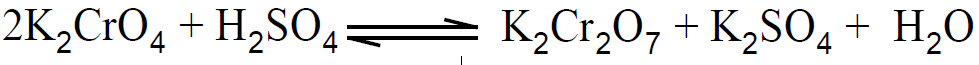
By adding more acid, equilibrium will shift towards the right side and more potassium dichromate will be produced.
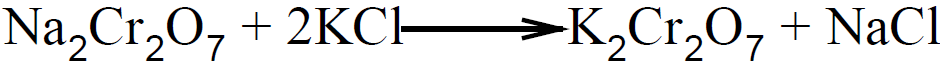
Q.9 Give systematic names of the following complexes.

Q.10 What are the typical and non-typical transition elements?
The elements of group IIB (Zn, Cd and Hg) and group IIIB (Sc, Y and La) are referred to as non-typical transition elements and the elements in the remaining groups of the transition series are called typical transition elements. Non-typical elements do not have partially filled d-orbital.
Q.11 Give a reason for the development of colours in the transition complexes.
In transition elements, the d-orbitals are responsible for the colour development in their compounds. When these orbitals are involved in bonding, they split up into two energy levels; one set has a higher energy than the other. The electron residing in low-energy d-orbitals absorbs a part of the visible light and jumps to high-energy d-orbitals. This process is called d-d transition. The energy difference of d-orbitals varies from ion to ion.
Thus every ion absorbs a different wavelength and transmits the remaining set of wavelengths that gives different colour to the ions.
E.g. In [Ti(H2O)6]3+ ion, yellow light is absorbed while most of blue and red light is transmitted, therefore its solution colour looks violet.
Q.12 How does K2CrO4 act as an oxidizing agent in the presence of H2SO4?
Dichromates are very powerful oxidizing agents. Oxidation is carried out in the presence of an acid. In this process, hexavalent chromium ion is reduced to trivalent chromium ion.
i.Ferrous sulphate oxidizes to ferric sulphate
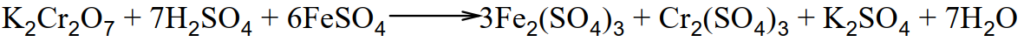
ii. KI oxidize to iodine
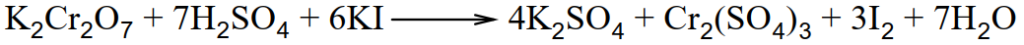
Q.13 How the entrapped gases are removed from the steel?
In order to remove entrapped bubbles of gases, such as O2, N2, and CO2 a little aluminium or Ferro-silicon is also added. Aluminium removes nitrogen as nitride.

Q.14 Explain the chromyl Chloride test and give its equation.
When solid potassium dichromate is heated with solid metal chloride in the presence of conc. H2SO4, chromyl chloride is produced.
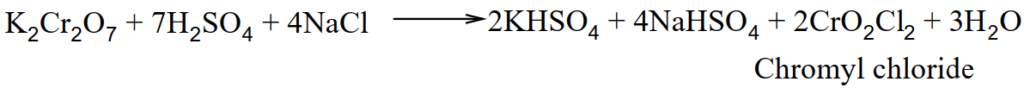
Q.15 What is the cause of paramagnetic behaviour?
Paramagnetic behaviour is caused by the presence of unpaired electrons in an atom, molecule or ion because there is a magnetic moment associated with the spinning electrons. It increases with the increase in the number of unpaired electrons.
Q.16 Give important characteristics of the transition elements.
- Melting and Boiling Points:
Transition elements have very high melting and boiling points due to strong binding forces present between their atoms. - Binding Energy:
Transition metals are tough, malleable and ductile. The toughness of these metals indicates strong metallic bonding. This is because, apart from s electrons of the outermost shell, the electrons of the underlying half-filled d-orbitals also participate in binding. - Oxidation State:
Transition metals exhibit variable valency or oxidation state. They show variable valencies because of the involvement of unpaired d-electrons to s-electrons in bond formation.
Q.17 Write down the uses of KMnO4.
- It is used as an oxidizing agent.
- It is used as a disinfectant and germicide.
- It is used in the manufacturing of many organic compounds.
Q.18 Define corrosion.
Any process of chemical decay of metal is due to the action of the surrounding medium is called corrosion.
Q.19 What are interstitial compounds?
When small non-metal atoms like H, B, C and N enter the interstices of transition metals and impart useful features to them, they are called interstitial compounds. These are non-stoichiometric compounds. Sometimes they are also termed interstitial alloys.
Q.20 Why d-block and f-block elements are called transition elements?
d-block and f-block elements are called transition elements because they are located between the s and p-block elements and their properties are in transition between the metallic elements of the s-block and non-metallic elements of p-block.
Q.21 Write two uses of K2Cr2O7.
- Potassium dichromate finds extensive use in dyeing.
- It is used in leather industries for chrome tanning.
- It is used as an oxidizing agent.
Q.22 What are paramagnetic and diamagnetic substances?
Substances which are weakly attracted towards a strong magnetic field are called paramagnetic.
Those substances which are weakly repelled by a strong magnetic field are called diamagnetic substances.
Q.23 What is medium carbon steel? Also, write its uses.
Medium carbon steel contains 0.2 -0.7% carbon. It is harder than mild steel. It is also malleable and ductile. It is used in making rails, axles and castings.
Q.24 Write down the types of ligands.
Ligands may be anions or neutral molecules e.g. K4[Fe(CN)6] and [Ag(NH3)2]Cl. In these examples, CN– is an anion ligand while NH3 is a neutral ligand.
According to the donation of lone pair, these are classified as
1- Monodentate (having a single donor atom)
e.g. NH3
2-Bidentate(having two donor atoms)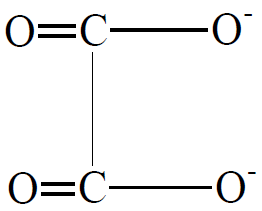 (oxalate anion is a bidentate ligand)
(oxalate anion is a bidentate ligand)
3-Tridentate (having three donor atoms) and
4- Polydentate (having many donor atoms)