Chemical bonding is one of the most important chapters in chemistry, It comprises all topics from an examination point of view. Mostly short questions/answers are given to cover the whole chapter but you combine a few short answers of the same topic to write an extensive answer. .
Chemical Bonding
Q. What is Chemical Bond?
The strong force of attraction that binds two or more atoms together to form variety of compounds.
Q. Write down the Causes of Chemical Bond Formation
- To attain stable electronic configuration (like noble gases)
- To lower their energy by bond formation
Q. Define Octet Rule.
The tendency of elements to attain maximum of eight electrons in their valence shell like noble gases (except He).
11Na = 1s2, 2s2,2p6, 3s1 → Na+ = 1s2, 2s2,2p6 like 10Ne
17Cl = 1s2,2s2,2p6,3s2, 3p5 → Cl-1 = 1s2, 2s2, 2p6, 3s2, 3p6 like 18Ar
Conclusion:
- Octet rule is not the only reason for the bond formation.
- Compounds like SF6, AlF3, PCl5 are exceptions of octet rule.
ENERGETICS OF BOND FORMATION
Potential energy and Internuclear distance
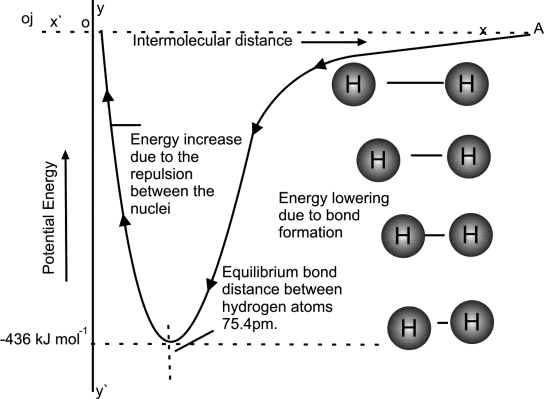
- Potential Energy is directly related to internuclear distance
- Bond distance / Bond length / Compromise distance is the minimum distance between bonded atoms.
- Bond formed when attractive forces between the atoms dominate the repulsive forces.
ATOMIC SIZE
Types:
- Atomic radius
- Ionic radius
- Covalent radius
Q. What are the techniques to measure the atomic size.
- By X-ray studies
- By spectroscopic measurements
Q. Define Atomic radius
The average distance between the nucleus and the outermost shell is called atomic radius.
Q. Write down the Factors affecting the atomic radius.
- Nuclear charge: Atomic size is inversely related to nuclear charge.
- Shielding effect: Atomic size is directly related to the shielding effect.
Q. Write periodic trends of atomic radius.
- Left to right in a period decreases
- Top to bottom in a group increases
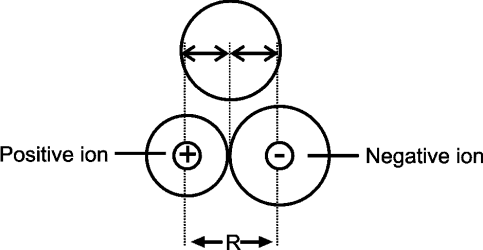
Ionic radius
Q. Define ionic radius.
The radius of an ion considering it to be spherical in shape.
Q. What are periodic trends of ionic radius?
- Left to right in a period decreases
- Top to bottom in a group increases
Q. Write down types of Ionic radii.
- Cationic radius
The radius of positively charged ion
The radius of cation is always smaller than its parent atom - Anionic radius
The radius of negatively charged ion
The radius of anion is always greater than its parent atom
Covalent radius
Q. Define covalent radius.
The half of the bond length between two covalently bonded similar atoms
Q. Write down the periodic trends of covalent radii.
- Left to right in period decreases
- Top to bottom in group increases
IONIZATION ENERGY
Q. Define ionization energy.
The minimum amount of energy required to remove an electron from isolated gaseous atoms to form ions.
Example: H(g) → H+(g) + 1e– ∆H = +1313.315 kJ/mole
Q. Factors affecting the ionization energy?
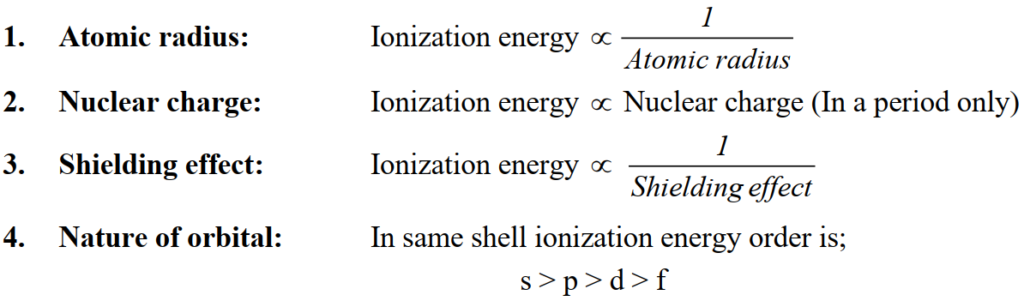
Q. Write Trends in the periodic table
- Left to right in period increases
- Top to bottom in group decreases
- Abnormal trend: Group III-A and VI-A elements show abnormal trend.
Q. What is the role of Ionization energy to determine valency.
- If there is big difference between 1st and 2nd I.E of an element, then valency is one and element belongs to group I-A.
- If there is big difference between 2nd and 3rd I.E of an element, then valency is two and element belongs to group II-A.
- If there is big difference between 3rd and 4th I.E of an element, then valency is three and element belongs to group III-A and so on.
Q. What is the role of Ionization energy to determine metallic character?
- Elements with lowest ionization energies are metals. (Left side of periodic table)
- Elements with Intermediate ionization energies are metalloids. (In the middle)
- Elements with greatest ionization energies are non-metals. (Right side of periodic table)
ELECTRON AFFINITY
Q. Define electron affinity.
The amount of energy released when an electron is added to the valence shell orbital of an isolated gaseous atom to form uni-negative ion.
Example: Cl(g)+1e– → Cl– (g) ∆He = -349 kJ/mole
Q. Write down the Factors affecting ionization energy
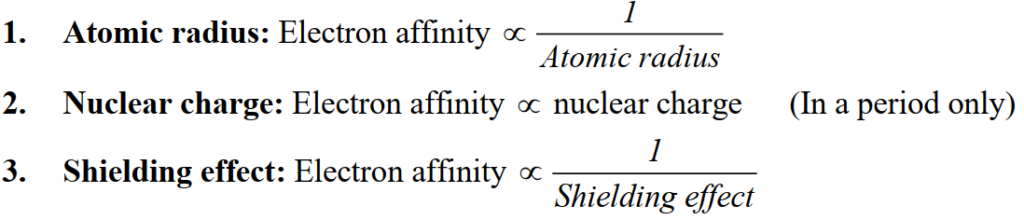
Q. Write down the Trends in periodic table of ionization energy.
- Left to right in period increases
- Top to bottom in group decreases
- Abnormal trend: Group-IIA, VA and VIIIA show the abnormal trend
ELECTRONEGATIVITY
Q. Define electronegativity.
The ability of an atom to attract shared pair of electron towards itself is called electronegativity.
Q. How does electronegativity determine the type of bonds.
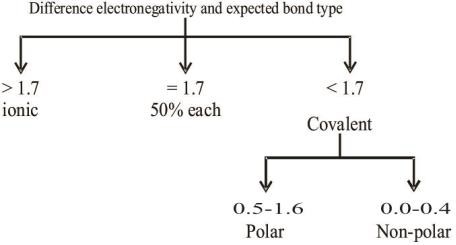
Q. Write down the periodic trend of electronegativity.
1. Left to right in period increases
2. Top to bottom in group decreases
Q. Why the size of a cation is smaller as compared to its parent atom?
Ans: The size of a cation is smaller as compared to its parent atom due to following reasons.
- When an atom loses its valance electrons, its valance shell vanishes and the size of cation become smaller
- By the loss of valance electrons number of protons become greater than the number of electrons so force of attraction toward the nucleus becomes stronger and the size of cations contracts i.e. decreases than its parent atom.
Q. Define ionization energy. Why the second ionization energy is greater than fist ionization energy?
Ans: The minimum amount of energy required to remove an electron from isolated gaseous atoms to form ions.
First electron is removed from the neutral atom so less energy is required whereas the removal of electron from positively charged ion is difficult due to stronger nuclear attraction. Hence more energy is required to remove second electron. Hence second ionization energy is always greater than the first ionization energy of an element.
TYPES OF CHEMICAL BONDS
Types:
- Ionic bond (Electrovalent Bond)
- Covalent Bond (Electron pair Bond)
- Co-ordinate covalent bond (Dative Bond).
IONIC BOND
Q. Define ionic bond.
The electrostatic force of attraction between oppositely charged ions is called ionic bond.

Q. what are the conditions necessary for the formation of ionic bond:
- Cations are electropositive elements with low ionization energy like I-A and II-A
- Anions are Electronegative elements with high electron affinity like VI-A and VII-A
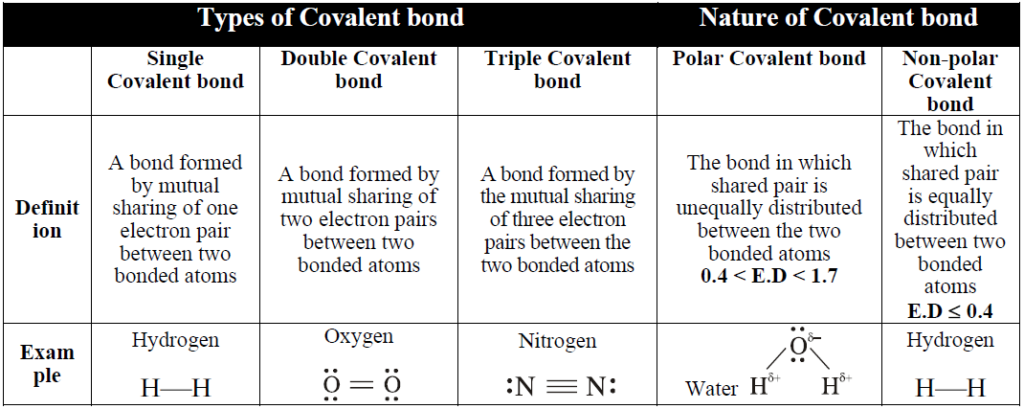
COVALENT BOND
Q. Define covalent bond.
A bond formed by mutual sharing of electron between the two atoms.
Example: Formation of Hydrogen-molecule. ![]()
COORDINATE COVALENT BOND
Q. Define coordinate covalent bond.
The covalent bond formed between two atoms in which shared pair of electrons is donated by one of the bonded atoms.
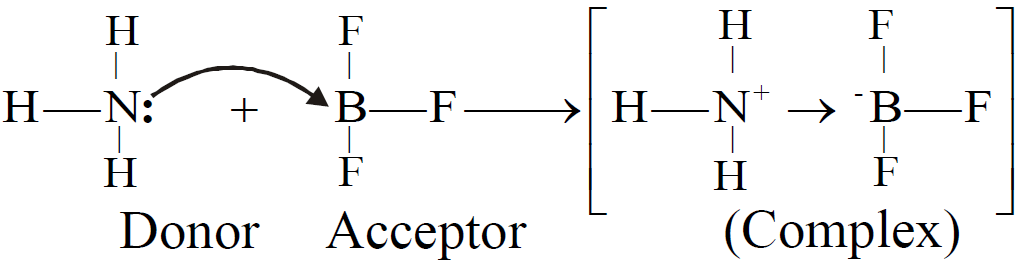
Q. Write down the Limitations of Lewis Model:
The following are the points that Lewis failed to explain.
- How electrons form pair despite of their repulsion.
- The molecular shapes.
- Molecular Geometries (Bond angles).
- Bond lengths.
- Bond polarities.
Q. No bond in chemistry is 100% ionic. Justify?
No bond in chemistry is 100% ionic because the maximum difference of electronegativity between the two bonded atom is 3.3 which is for CsF. According to Pauling’s formula the percentage ionic character for CsF is 92%. It means maximum value of ionic character for any bond in chemistry is 92%, so above statement is justified.
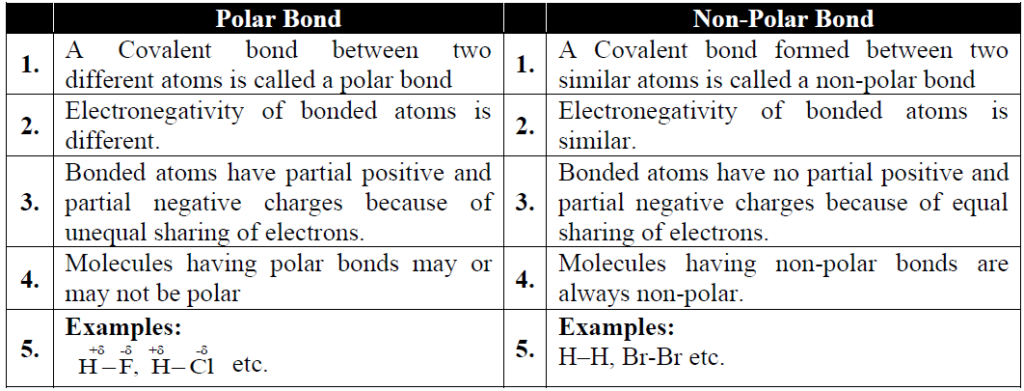
Q. Differentiate between non-polar and polar covalent bond. Give examples.
MODERN THEORIES OF COVALENT BOND
Modern theories gave satisfactory explanation of bond formation.
- Valence shell electron pair repulsion theory (VSEPR Theory).
- Valence bond theory (VBT) and Hybridization Theory (modified VBT).
- Molecular Orbital Theory (MOT).
VSEPR THEORY
Q. Write down the Basic assumption for VSEPR theory.
The electron pairs (lone pairs and bond pairs) arrange themselves around central atom at maximum distance apart so that repulsion between them is minimum.
Q. Write Postulates of VSEPR Theory:
- Both Lone pair and bond pair participate in determining the geometry of molecules.
- The electron pairs are arranged around central polyvalent atom at maximum distance apart to keep the repulsions minimum.
- The lone pair occupies more space than bond pair and hence, lone pair causes more repulsion than bond pair. The magnitude of repulsions is in the order of:
Lone-pair-lone-pair > Lone pair-bond pair > Bond pair-bond pair - Three electron pairs of triple bonds have higher electron density and occupy more space than two electron pairs of double bonds which in turn has higher electron density and occupy more space than single bond, but multiple bonds (double or triple bond) behave like a single bond in determining the geometry of molecule.
- The bond angle reduces with the increasing electronegativity of the bonded atoms.
Types of Molecules
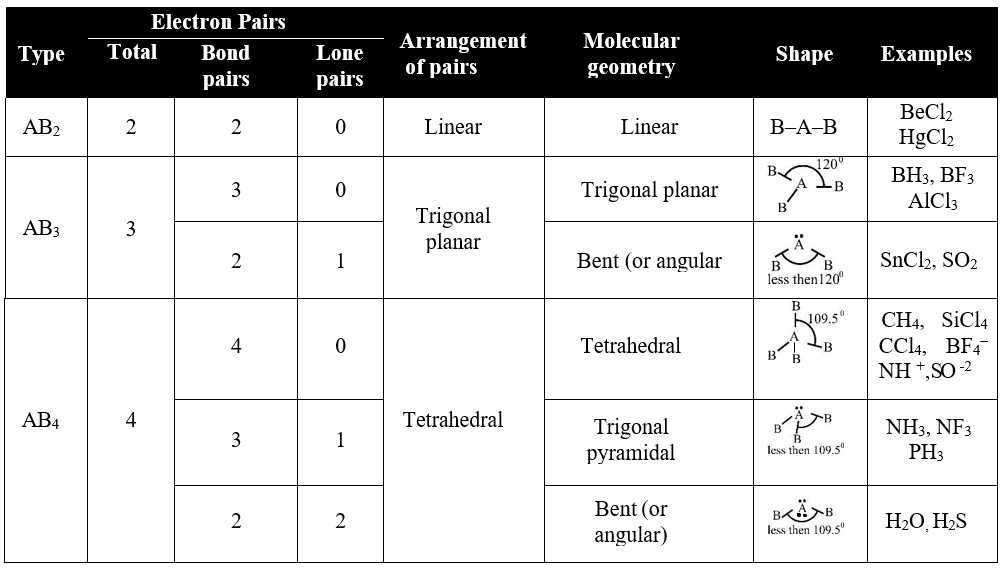
Q. Lone pair of electrons occupy more space as compared to bond pairs of electrons. Justify it.
The lone pairs occupy more space. It is due to
A lone pair experiences less nuclear attraction because it is bound to single nucleus while in bond pair electrons are under the influence of both nuclei.
Secondly electronic charge of lone pair is spread out more in space than that of bond pair and thus tend to compress the bond pairs and having more space than bond pair.
Q. Why NH3 is a pyramidal molecule.
Geometry of NH3

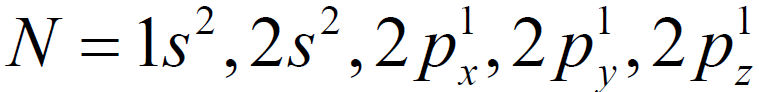
The non-bonding electron in 2s orbital takes up more space and exerts a strong repulsive force on the bonding electron pairs. Consequently, to avoid a larger repulsion, the bonding electron pairs move closer that reduces the ideal bond angle from 109.5° to 107.5°. This effect compels ammonia to assume a triangular pyramidal geometry instead of tetrahedral, as in methane.
VALENCE BOND THEORY
Q. Write Postulates of VBT.
- Overlapping is a necessary condition for bond formation.
- Only half filled or partially filled orbital participate in overlapping..
- Greater the overlapping, stronger is the bond formed.
- Electrons with opposite spin pair up to stabilize themselves during bond formation.
- Sigma (σ) bond: A bond formed by head-to-head overlapping.
- Pi (π) bond: A bond formed by the parallel or sideways overlapping.
Q. Write Limitations of VBT.
- It does not justify the valency of some elements i.e., Carbon, Beryllium, Boron etc.
- It does not explain equivalent tetravalency of carbon.
- It does not explain paramagnetic and diamagnetic behaviors of substances.
ATOMIC ORBITAL HYBRIDIZATION
Q. Define atomic orbital hybridization.
Hybridization is the process of mixing of atomic orbitals of different energy and shape to form new orbitals of same energy and same shape.
Q. What are basic types of atomic orbital hybridizations.
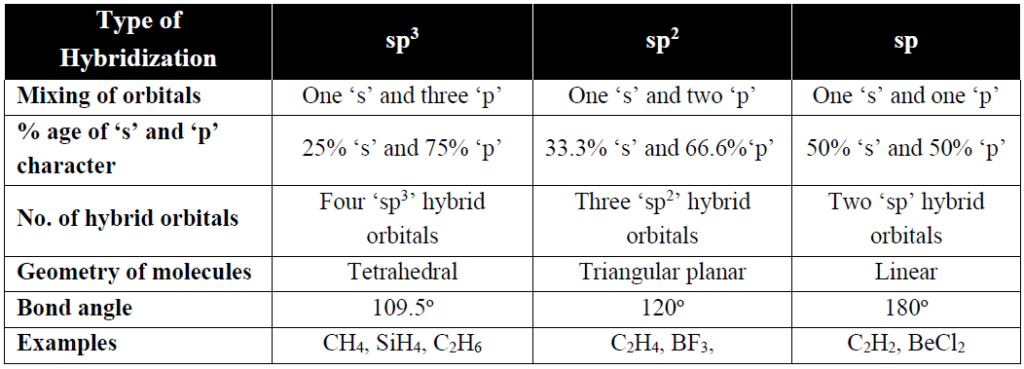
Q. Explain that the π-bonds are more diffused than σ-bond.
As sigma bond lies along the internuclear axis and under the strong hold of two bonded nuclei. While in the pi-bond the electron density lies away (above and below) from internuclear axis. That is why π-bonds are more diffused.
Q. The bond angles of H2O and NH3 are not 109.5˚ like that of CH4. Although O and N atoms are sp3 hybridized. Give reason.
As the O-atom of water has two lone pairs of electrons and N-atom of ammonia has one lone pair of electrons. Therefore, according to VSEPR theory the repulsion among the bond pairs and lone pairs of electrons decreases the bond angles from 109.5˚.
MOLECULAR ORBITAL THEORY
Q. Write down the Postulates of MOT.
- Valence atomic orbitals overlap to form molecular orbitals.
- The number of molecular orbitals equal to the number of atomic orbitals.
- Half of the molecular orbitals are called bonding molecular orbitals (BMO) while other half are anti-bonding molecular orbitals (ABMO).
- BMO formed by Linear overlaping are called sigma (σ) orbitals while ABMO are called sigma asteric (σ*) orbitals.
- The BMO formed by parallel overlapping are called pi ( π ) orbitals while ABMO orbitals are called as pi asteric ( π *) orbitals.
- The electrons are distributed in the molecular orbitals according to Aufbau principle, Pauli’s exclusion principle and Hund’s rule.
- The number of bonds between the two atoms is given by bond order.
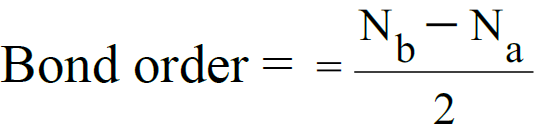
Nb = electrons in bonding molecular orbital.
Na= electrons in anti-bonding molecular orbital. Example:
Example:
- Formation of H2, O2 and N2 molecules
- Non-existence of He2, B2 etc.
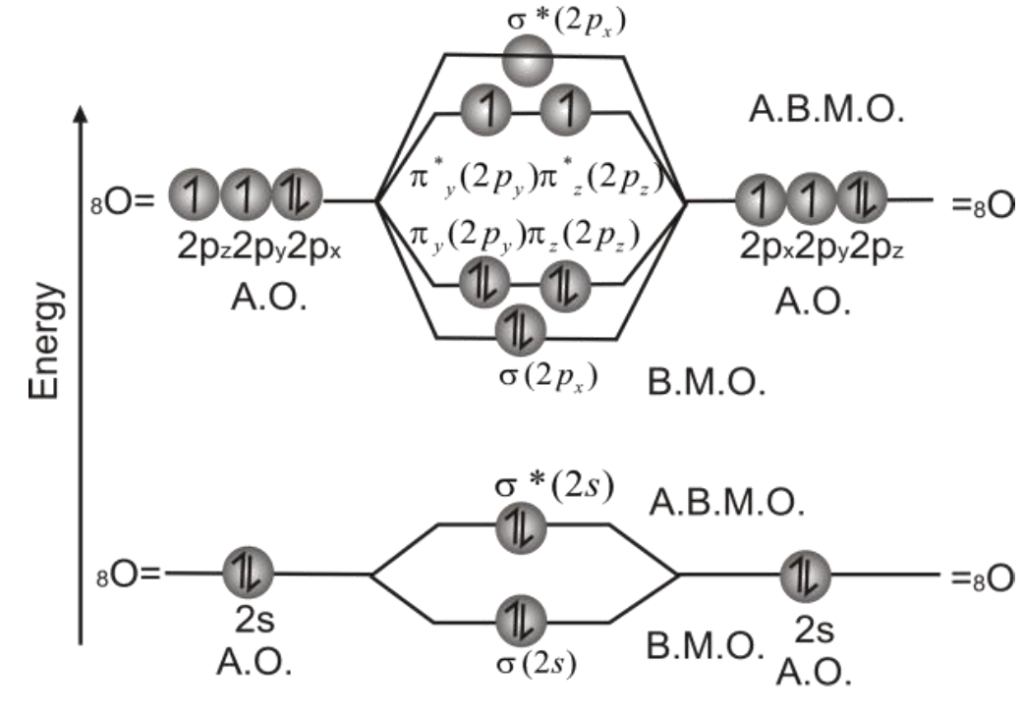
Q. Draw molecular orbital diagram of O2-molecule
1- Electronic configuration:
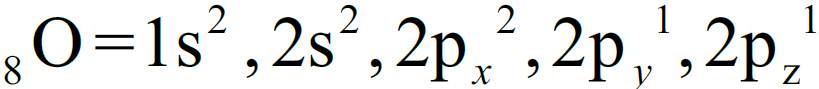
3- Molecular Orbital Diagram of Oxygen molecule is as follows.
3- Bond Order.
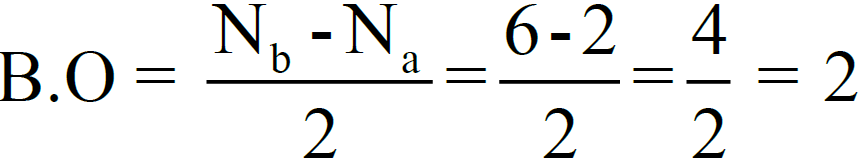
So, there are two bonds (double covalent bond) between two oxygen atoms (one σ– bond and one π-bond). The bond dissociation energy of O2-molecule is 495 kJ/mole.
Relative energy levels of molecular orbitals in O2 molecule:
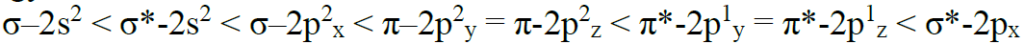
BOND ENERGY
Q. Define bond energy.
The average amount of energy required to break all the bonds of a particular type in one mole of a substance.
Q. Write down the factors effecting bond energy (BE):
- Size of atom: larger size lesser B.E
- Bond length: Smaller bond length greater B.E
- Electronegativity: Greater difference between bonded atoms, stronger bond
- Hybridization: sp – sp > sp2 – sp2 > sp3 – sp3
BOND LENGTH
Q. Define bond length.
The distance between the nuclei of two covalently bonded atoms.
Q. Which methods are used to measure bond length.
- Electron diffraction
- X-ray diffraction
- Spectroscopic studies
Q. Write Factors affecting bond length.
- Size of bonded atoms
- Nature of hybridization
- Electronegativity difference
DIPOLE MOMENT (μ)
Q. Define dipole moment.
The product of electric charge (q) and distance between positive and negative center (r)
Q. Calculate the units of dipole moment.
Dipole moment is a vector quantity.
The SI units of dipole moment is mC (1D = 3.336 x 10-30 mC) D = Debye
Q. How to calculate the Dipole moment for diatomic and polyatomic molecules?
In diatomic molecules (HF, HCl, HBr), dipole moment is calculated by formula μ = q x r.
Polyatomic molecules contain more than one dipole moment. Net dipole moment is the resultant of vector addition of individual dipole moments.
Dipole moment and molecular structure:
Q. how to calculate Percentage ionic character?
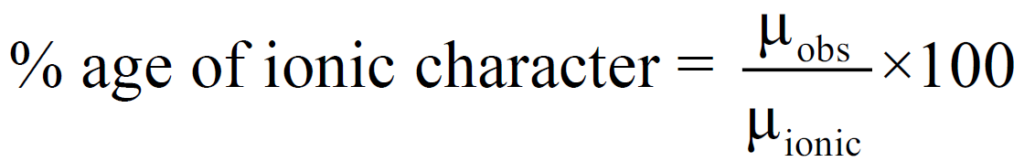
Q. What is the relationship between bond angle and geometry of molecules.
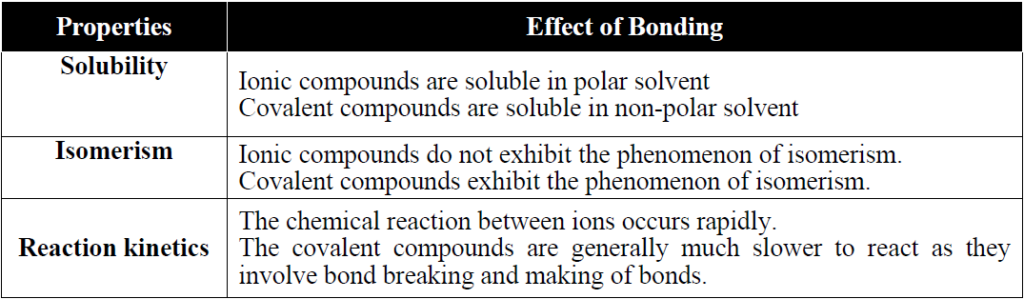
Q. What is EFFECT OF BONDING ON THE PROPERTIES OF COMPOUNDS
Q. Justify that molecular orbital theory is superior to valance bond theory.
MOT explains:
- Paramagnetic and diamagnetic behavior of molecules
- Existence of molecules
- The bond order of molecule
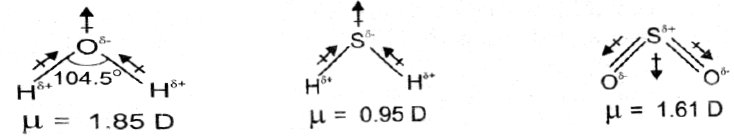
Q. The dipole moments of CH4 and CO2 are zero but that of H2O is 1.85D. Why?
Ans: Because in CH4 four valance electrons of carbon are covalently bonding with four neighboring hydrogen atoms and has regular tetrahedral structure and the some dipole moments becomes zero. In CO2-molecule central atom is carbon which has four electrons in its valence shell and form double bond with two oxygen atoms. Overall molecule is non-polar have zero dipole moment and have linear structure. Where as in water molecule central atom is oxygen which has two lone pair and two bond pairs in its valance shell, so due to the presence of two lone pair molecule have bent or angular structure its dipole moment is 1.85 D.
