Aromatic Hydrocarbons is the chapter that describes the chemistry of benzene and important derivatives of benzene. In our discussion benzene and monosubstituted benzene are taken for study. The whole chapter may be revised by learning short questions. A few short questions can be used collectively to make extensive questions.
INTRODUCTION TO AROMATIC HYDROCARBONS
Q1. Define Aromatic Hydrocarbons:
Hydrocarbons containing at least one benzene ring in their molecules are called aromatic hydrocarbons.
Q2. Write down the classification of aromatic hydrocarbons.
On the basis of the number of benzene rings, aromatic hydrocarbons are categorized into two
classes.
- Monocyclic Aromatic Hydrocarbons:
Containing one benzene ring in their molecules
Example:
Benzene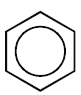
- Polycyclic Aromatic Hydrocarbons:
Containing two or more benzene rings in their molecules
a) Those in which benzene rings are isolated.
Example: Biphenyl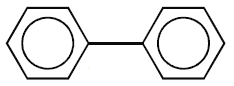
b) Benzene rings are fused together at ortho positions so that adjacent rings have common carbon
to carbon bonds.
Example: Anthracene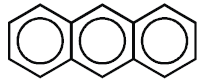
NOMENCLATURE OF AROMATIC HYDROCARBONS
Q3. Write down the rules for the nomenclature of aromatic hydrocarbons.
- Monosubstituted benzene derivatives are named by prefixing benzene by the name of the
substituent. The whole names are written as one word Example: Ethylbenzene - There are certain monosubstituted benzene derivatives which are given the special names
Examples: Benzoic acid, Aniline, Toluene, etc.
Aryl Group: When a hydrogen atom is removed from benzene, we get a phenyl group
symbolized by C6H5- or ph. C6H6 → C6H5–
Substituted phenyl groups are called aryl groups.
Polysubstituted Benzene Derivatives: - The second substitution in benzene would give rise to three isomeric products designated as ortho
(1,2), meta (1,3) and para (1,4). - If two or more substituents are different, then the substituent that is treated as a high-priority
group is given the number 1 position in the benzene ring. Other groups are numbered by
counting them from position 1 in the manner that gives them the lowest number.
The order of priority of the group (left to right) is as follows:
―COOH, ―CN, ―CHO, ―COCH3, ―OH, ―NH2, —OR, —R
Examples: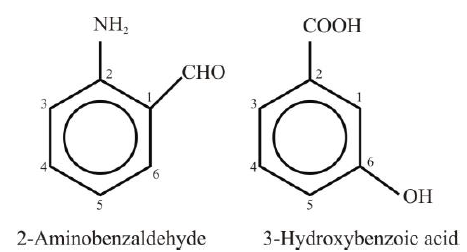
- If the two substituents are different and they are not present in priority order, then use
alphabetical order. The last named substituent will be at position 1.
Example: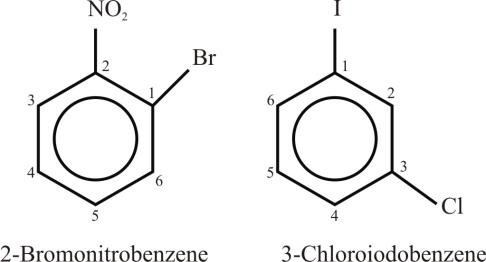
- If there is a substituent on the ring that gives a special name to the molecule, then a special name is used as a parent name to the molecule
Example: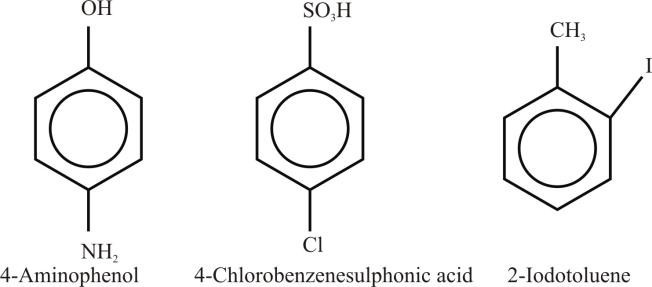
BENZENE
Q4. Write a few lines on the Structure of Benzene:
a) The empirical formula of benzene is determined by chemical analysis. Its empirical formula is CH.
b) The molecular mass of benzene is determined by the vapour density method which shows that
the molecular mass is 78.108.
c) The molecular formula of benzene indicates that it is a highly unsaturated compound.
Q5. How is the straight Chain Structure of benzene Ruled Out?
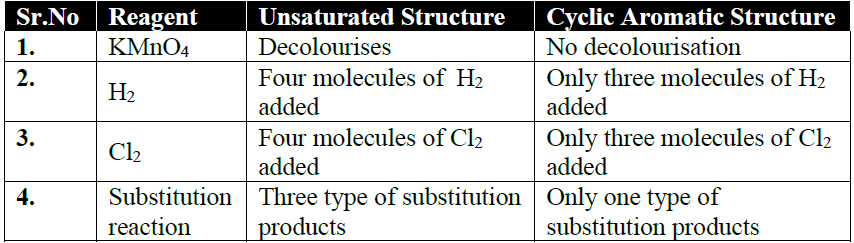
Q6. How Kekule described the structure of benzene.
Benzene has a cyclic regular hexagonal structure
Kekule structure is supported by the following facts
- Benzene gives only one mono-substituted product.
- Benzene gives three isomeric disubstituted products.
- Benzene adds three molecules of H2 in the presence of a catalyst.
- Benzene adds three molecules of chlorine in the presence of sunlight.
NOTE: Points 3. and 4. indicate the presence of three double bonds in a molecule of benzene.
Q7. What information do we get from X-ray analysis of benzene?
X-ray studies show that benzene has a planar cyclic regular hexagonal uniform structure. All the angles are 120o, the C-C bond length is 1.397Ao and the C-H bond length is 1.09Ao. 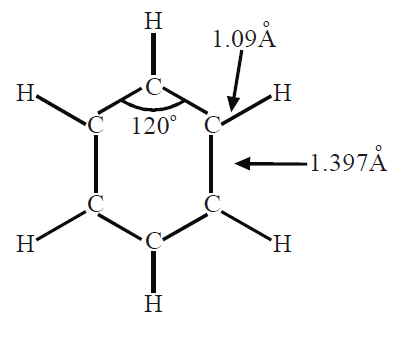
Q8. What was the objection to Kekule’s structure of benzene?
According to Kekule’s formula benzene contains three double bonds, therefore it should behave like a highly unsaturated compound whereas benzene usually shows a saturated character. It gives substitution reactions readily and forms addition products reluctantly which indicates that benzene is a very stable compound.
Q9. Write about the modern concepts of the structure of benzene.
Following is the atomic orbital Treatment of Benzene.
- Hybridization
Every carbon in benzene is sp2 hybridized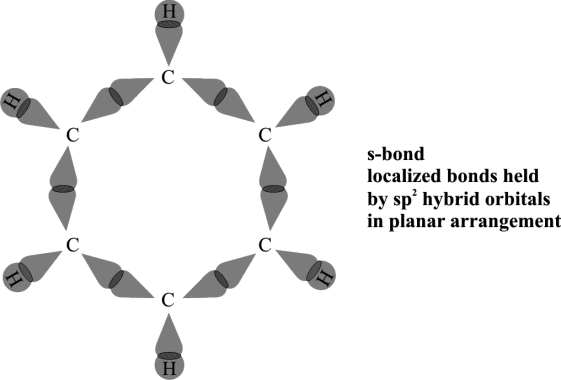
- Unhybridized Orbitals
Every carbon in benzene has one 2pz unhybridized orbital when they overlap in a parallel manner they form three pi bonds.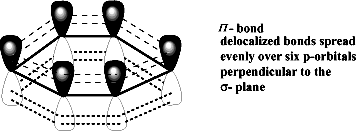
- Continuous Sheath of Electron Cloud.
A continuous sheet of electrons above and below the carbon atoms of benzene makes it very stable.
Q10. How the Stability of benzene is proved.
The stability of benzene is due to the extensive delocalization of the electronic cloud.
Q11. Write resonance method for benzene.
Resonance: The possibility of electrons pairing in unhybrid 2p orbitals is called resonance. Resonance is represented by ↔.
Various Resonance Structures Proposed by Kekule and Dewar
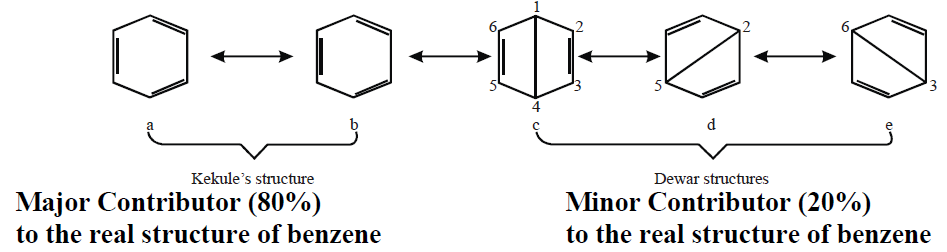
Conclusion:
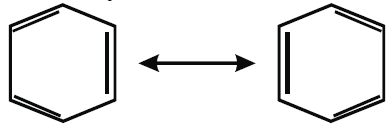
The benzene molecule is usually represented by any of the following Kekule’s structures.
Q12. Write down the methods of preparation of benzene.
1- From Coal and Petroleum
Benzene and other aromatic hydrocarbons are readily obtained in large quantities from Coal by
its destructive distillation.
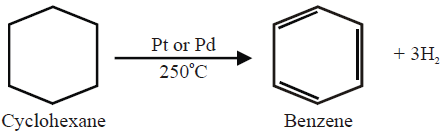
2- Dehydrogenation of Cyclohexane:
3- From Acetylene:
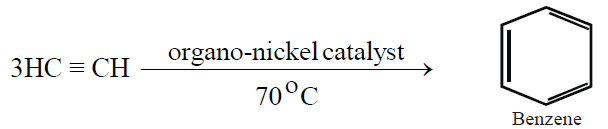
4- From Alkanes:
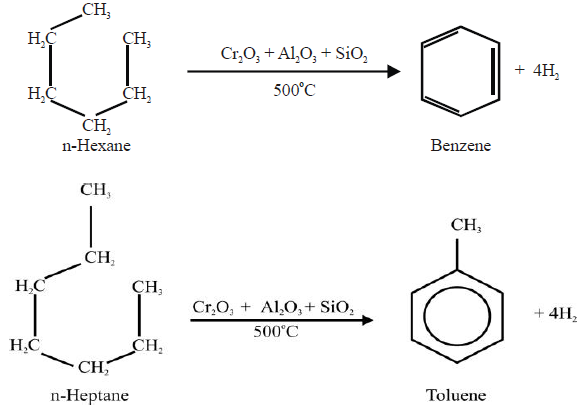
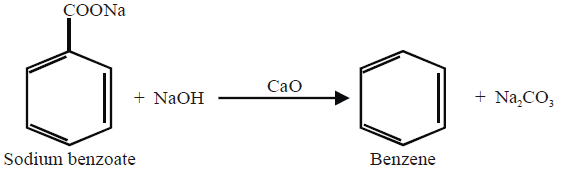
5- From Sodium Benzoate: (Lab Method)
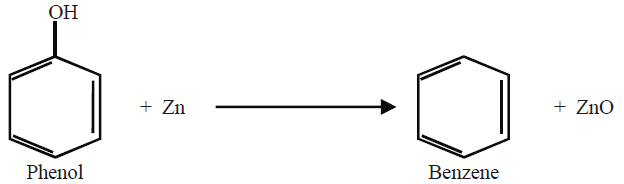
6- From Phenol: (Lab Method)
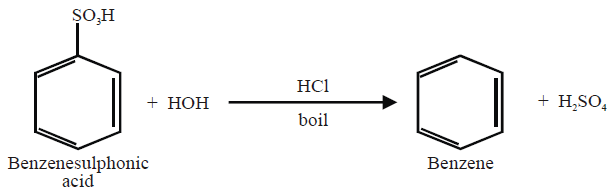
7- From Benzene sulphonic Acid: (Lab Method)
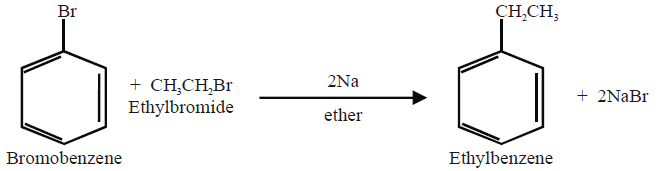
Q13. Write Wurtz-Fittig Reaction:
ELECTROPHILIC SUBSTITUTION REACTIONS OF BENZENE
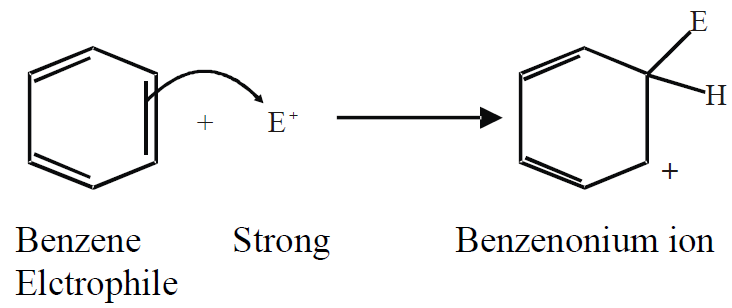
Q.14: Write down the general mechanism for the electrophilic substitution reaction of benzene.
In electrophilic substitution reactions, benzene reacts with strong electrophiles.
a) Attack of electrophile (on π-electrons of benzene):
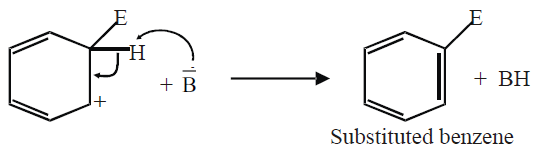
b) Departure of hydrogen:
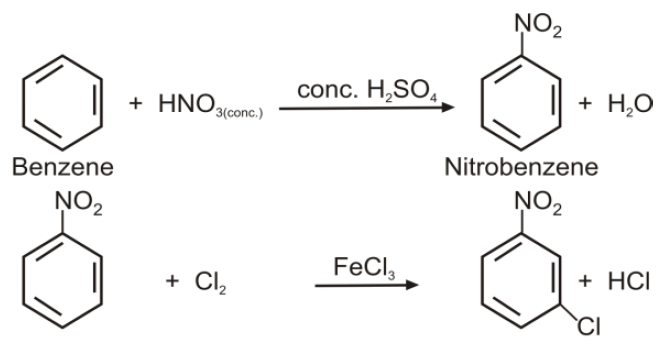
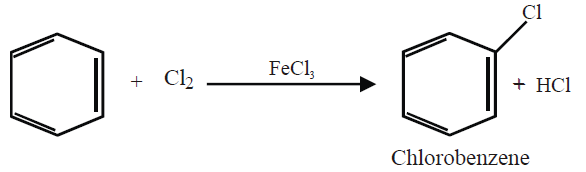
Q15. Write down the Halogenation of benzene.
Substitution of a hydrogen atom by halogen group in the benzene ring.
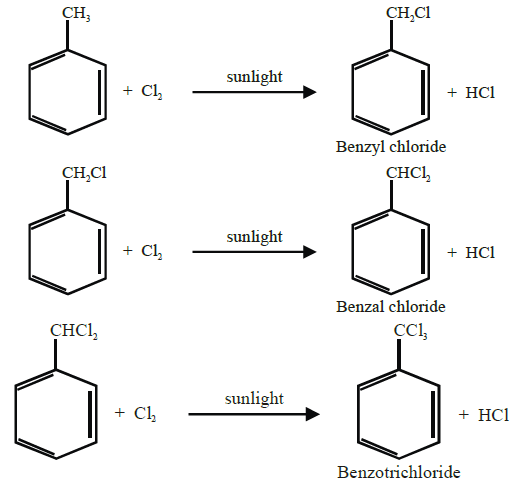
Side chain halogenation (by substitution):
When alkyl benzenes are treated with chlorine or bromine in the presence of sunlight, only the
alkyl groups are substituted.
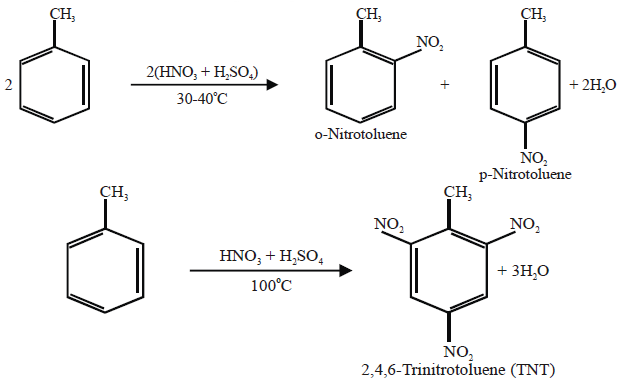
Q16. Write nitration in benzene.
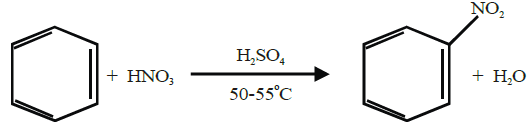
The substitution of a hydrogen atom by nitro group (-NO2) in the benzene ring is called nitration.
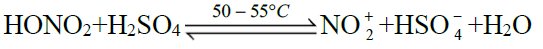
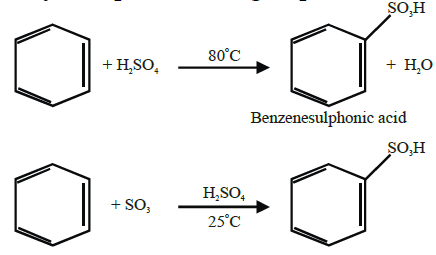
Q17. Write sulphonation of benzene.

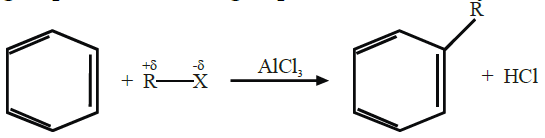
Q18. Describe Friedel Craft’s Reactions.
Friedel Craft’s reactions are of two types.
1- Friedel Craft’s Alkylation

2- Friedel Craft’s Acylation.
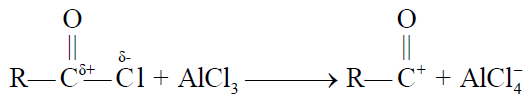
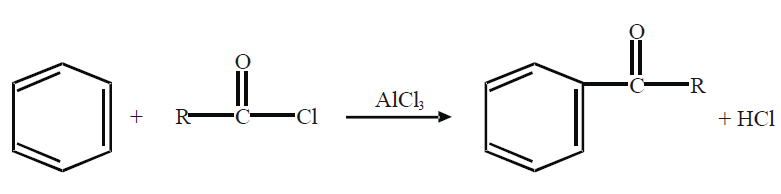
REACTION IN WHICH BENZENE RING IS INVOLVED
Q19. Write Important chemical reactions of Benzene
1- Addition Reactions:
a. Reduction
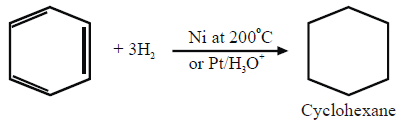
b. Halogenation:
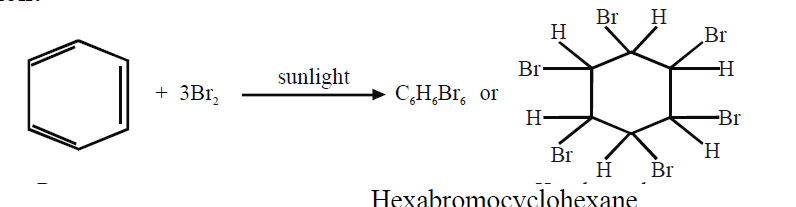
2- Combustion
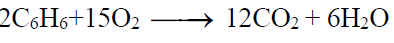
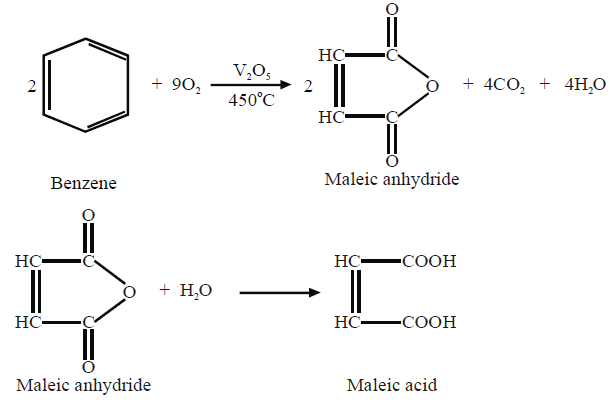
3- Oxidation
i. Catalytic Oxidation.
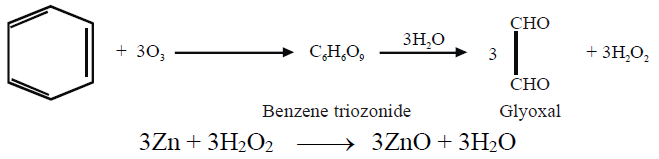
ii- Ozonolysis
Side Chain Reaction
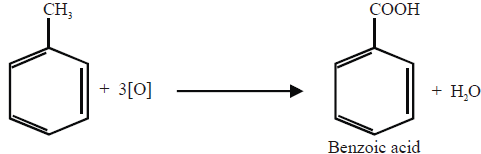
ORIENTATION IN ELECTROPHILIC SUBSTITUTION REACTIONS
Q20. What is orientation in benzene?
The introduction of the second group into the benzene ring is directed (orientated) according to the 1st group already present on the benzene ring. Benzene may give three isomeric disubstituted products which are ortho, meta and para.
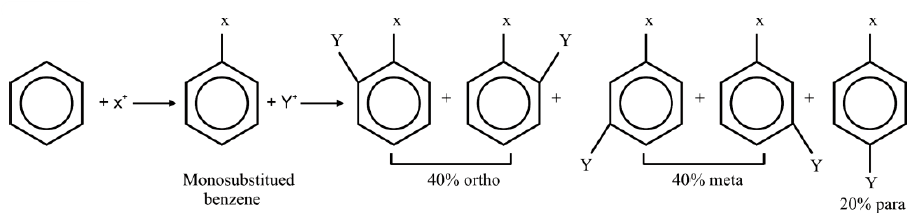
On a chance basis 40% ortho, 40% meta and 20% para-disubstituted products are expected.
Ortho-and para-directing groups:
These groups release electrons to the benzene ring thereby facilitating the availability of
electrons to the electrophiles at the ortho and para positions.
Example: —N(CH3)2, ― NH2 , ― OH, ― OCH3 , ― Cl, — Br, — I
Meta-Directing Groups:
When the electron-withdrawing group is present on the benzene ring then it will deactivate ortho, para position
and comparatively meta position has an excess of electrons so the incoming group will attach at meta position.
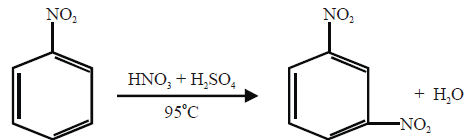
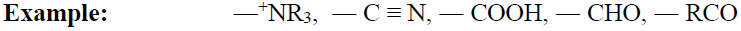
Q21. How will you prepare m-Chloronitrobenzene from benzene in two steps?