41. 11g of carbon is reacted with 32g of O2 to give CO2. Which one is the limiting reactant?
C+O₂ → CO₂
According to balanced chemical equation C = 1mole, O₂ = 1mole & CO₂= 1 mole
But we are provided with
![]()
![]()
Hence “C” has less the number of moles. So, it is a limiting reactant.
42. Give two examples of compounds having the same Empirical and Molecular formula?
Following are some examples, which have some empirical and molecular formulas.
- H2O(Water)
- NH3 (Ammonia)
- CO₂ (Carbon dioxide)
- C12H22O11 (Sucrose)
43. 23g of Na and 238g of U have equal numbers of atoms in them. Justify?
Molar Mass of Na = 23 g/mole & Molar mass of U = 238g/mol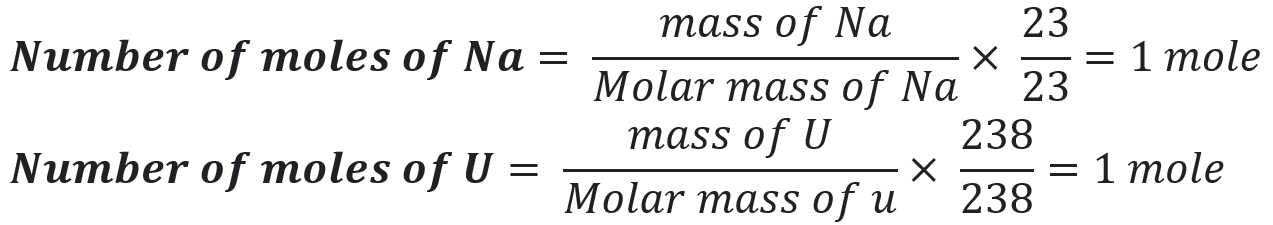
Since one mole of each element contains 6.02×1023 particles. So, both have an equal number of atoms in them.
44. Many chemical reactions in our surroundings show limiting reactants. Explain with an example.
Example: Burning of coal:
In the burning of coal oxygen (O2) and coal (C) are reactants. As oxygen is present in an excess amount so coal (C) is our limiting reactant.
C+O₂(excess) → CO₂
45. Write down the names of the water absorber and CO2 absorber in the combustion analysis experiment.
H2O absorber is Mg(CIO4)2 & CO2 Absorber is 50% KOH.
46. Calculate the mass in Kg of 2.6 x 1020 molecules of SO₂?
| No. of molecules of SO2(N) | = 2.6×1020 |
| Molar mass of SO2 (M) | = 32+(16+16) =64 g/mole |
| Mass in Kg of SO₂ (m) | =? |
| m | = (N × M)/NA |
| = 27.64 × 10-3 g | |
| = 27.64× 10-6 Kg |
47. One Mole of water has two moles of bonds, and three moles of atoms. Explain?
Each molecule of water has two bonds; therefore, one mole of water must contain double the number of moles of bonds.
Each molecule of water contains three atoms (2 Hydrogen & one Oxygen), therefore one mole of water must have three times the number of moles of atoms.
48. Define Isotope. Give an example.
Atoms of the same elements can possess different atomic masses but the same atomic numbers, such atoms of an element are called isotopes. For example, carbon has three isotopes written as C-12, C-13 and C-14.
49. Define atomicity? Give two examples.
The number of atoms present in a molecule is called atomicity. For example, the atomicity of H2O is three and CH4 is five etc.
50. Calculate the number of moles of O atoms in 9.00 grams of Mg(NO3)2?
| Formula mass of Mg(NO3)2 | =24+(14×2+16×6) =148 g/mol |
| Moles of Mg(NO3)2 | = 9g/148gmole-1 = 0.06mol |
| 1mol of Mg(NO3)2 has moles of O atoms | = 6mole |
| 0.06 moles of Mg(NO3)2 has | = 6 x 0.06 = 0.36 mol |




