FSc. Class 11 Chapter 4 comprises two sections 1st is liquids and second is solids. The ‘solid’ section is discussed as follows and notes of ‘Liquids’ are here.
SOLIDS
That substance has a definite volume and definite shape.
Example: NaCl, Diamond etc.
What are cohesive forces?
Forces present between particles are called cohesive forces.
TYPES OF SOLIDS
1. Crystalline Solids:
Constituent particles are arranged in a definite three-dimensional pattern.
Example: NaNO3, KNO3.
2. Amorphous Solids: (Pseudo solids)
Constituent particles do not possess a regular orderly arrangement.
Example: Glass, Plastics, Rubber, Glue etc
Define crystallites.
The orderly arrange part of amorphous solids.
What are the properties of amorphous solids:
- No sharp melting points.
- No definite heats of fusion.
- It may have hardness and elasticity.
- Isotropic in nature.
- No fixed geometrical shape.
How does the conversion of crystalline compounds into amorphous is carried out?
By melting crystalline compound then suddenly cooled
Write down the properties of crystalline solids.
- Geometrical Shape: Definite, distinctive.
- Melting Points: Sharp melting points.
- Cleavage Planes: Direction through which a crystal can be broken.
- Anisotropy: The property which depends upon the direction of the crystal.
Name the Anisotropic properties.
- Refractive index
- Co-efficient of thermal expansion
- Electrical conductivities
- Thermal conductivities
- Cleavage of crystalline solids
Define symmetry and its elements.
Repetition of faces, angles or edges when a crystal is rotated by 360o along its axis.
Symmetry elements:
- Centre of symmetry
- Plane of symmetry
- Axis of symmetry
Define the Habit of a Crystal.
The shape of a crystal in which it grows is called the habit of crystal.
Example: The habit of the crystal of NaCl in the pure state is to make cubic crystal.
What is the effect of impurity on the habit of crystal?
NaCl generates needle-like crystals when 10% urea is present in its solution as an impurity.
Define isomorphism.
Existence of two or more substances in the same crystalline form.
Examples:
| (i) NaNO3 and KNO3 | Rhombohedral |
| (ii) K2SO4 and K2Cr2O4 | Orthorhombic |
Define Polymorphism
Existence of a substance in more than one crystalline form.
Examples.
| (i) CaCO3 (ii) AgNO3 | Orthorhombic, Rhombohedral or Trigonal Rhombohedral, Orthorhombic |
Define allotropy.
Existence of an element in more than one crystalline form.
Example
| (i) Sulphur (ii) Carbon | Rhombic, monoclinic Graphite (hexagonal), Diamond (cubic) |
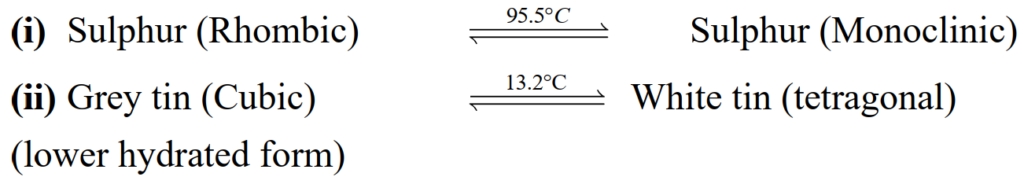
Define transition temperature.
The temperature at which a substance’s two or more crystalline forms co-exist in equilibrium.
Example
Cleavage of crystal is itself anisotropic behaviour. Justify it
Whenever the crystalline solids are broken, they do so along definite planes. These are called cleavage planes and are inclined to one another at a particular angle for a given crystalline solid. Due to this reason, the cleavage of crystals is known as anisotropic property.
An amorphous solid like glass is also a super-cooled liquid. Why?
The arrangement of the constituents of amorphous solids is like that of liquids i.e. no
particular arrangement. Thus, glass which is solidified without any arrangement and softens over a range of temperatures is an amorphous solid called super-cooled liquid.
Define Crystal lattice.
The particular three-dimensional arrangement of particles in a crystal.
Define Lattice Point or Lattice Sites
Space, points or position occupied by the particles in a crystal lattice is called lattice points.
Define unit cell
The smallest part of a crystal describes the characteristics of the entire crystal lattice.
What is the significance of the study of a unit cell?
- Smallest block/geometrical figure of a crystal.
- The entire crystal can be built up by repeating it.
- Describes particle arrangement in whole crystal.
- Quantitative aspects are deduced from the size and shape of a unit cell.
What are unit cell dimensions or crystallographic elements?
Three unit cell lengths a, b and c and three unit cell angles ∝, β and ⋎ are called crystallographic elements.
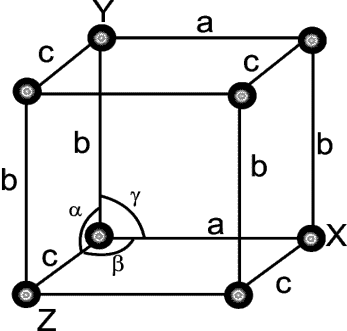
Classify seven crystal systems.
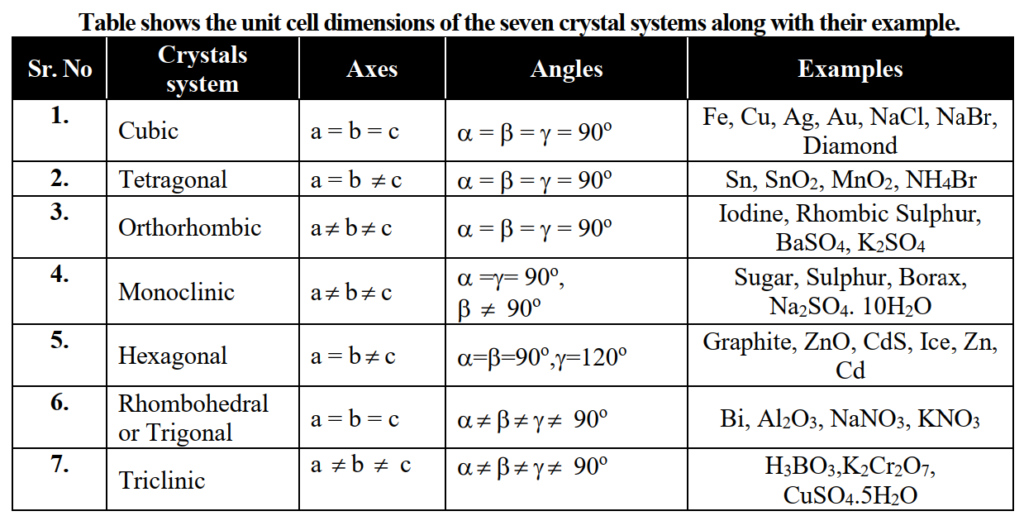
Draw the shape, axes and angles of the hexagonal system.
In a hexagonal system, two axes are of equal length and are in one plane making an angle of 120° with each other. A regular polygon always has equal angles. A hexagonal crystal is a regular six-sided polygon so its angles are equal to 120º. The third axis which is different in length than the other two is at a right angle to these two axes.
Sodium chloride and cesium fluoride have the same geometry, comment on it.
Sodium chloride and cesium fluoride have the same cubic geometry in crystals
because the ratio between their cation and anion sizes is almost the same. In sodium chloride sodium ion is smaller than chloride ion while in cesium fluoride, fluoride ion is smaller than cesium ion.
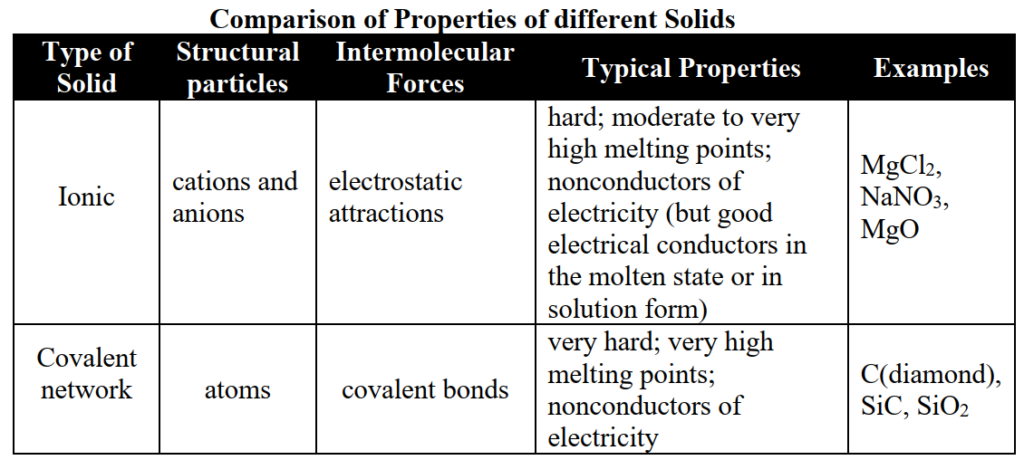
Write down the classification of solids on the bases of cohesive forces.
- Ionic solids
- Covalent solids
- Molecular solids
- Metallic solids
Define ionic solids.
Those solids in which ions are held together by electrostatic forces / ionic bonds are called ionic solids.
Example: NaCl, KBr, etc
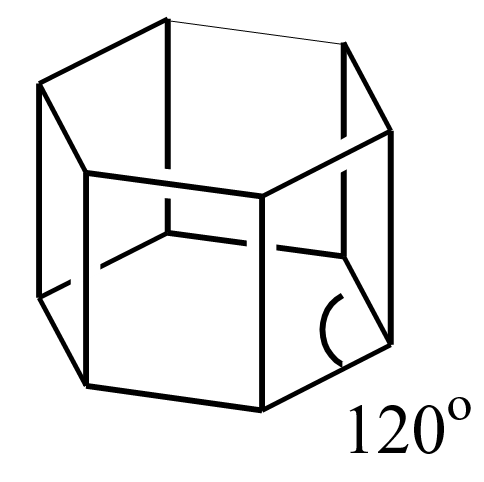
Briefly explain the structure of sodium chloride.
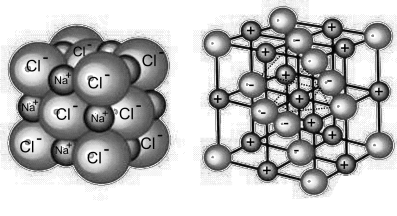
- Size of Na+ and Cl– ions: The size of Cl– is bigger than the Na+ ion.
- Inter-ionic distance (between Opposite ions): 2.815Ao.
- Inter-ionic distance (between same ions): 5.63Ao.
- Co-ordination Number
The number of ions of the same kind that surround an oppositely charged ion is called the coordination number.
Co-ordination No. of Na+ is 6
Co-ordination No. of Cl– is 6 - Formula unit: 8/8 + 6/2 = 4 Cl– ions
- Lattice Energy:
The amount of energy released when one mole of a crystalline solid is formed from the gaseous ions.
Example: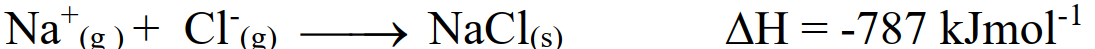
What are covalent solids?
Those solids in which neutral atoms (similar or different) are held together by covalent bonds to form a covalent lattice or atomic solids.
Examples: Diamond, graphite, Silicon carbide (SiC), Aluminium nitride (AlN) etc.
Types of covalent solids.
- Giant covalent solids: e.g. Diamond, SiC, AlN
- Layered covalent solids: e.g Graphite, BN, CdI2
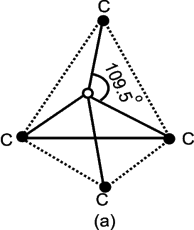
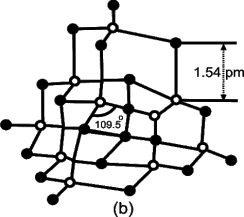
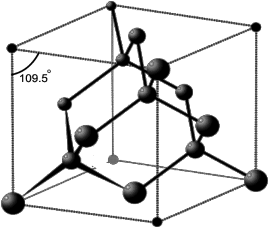
Write structure of diamond.
Each carbon atom is linked with four other carbon atoms. The four atomic orbitals undergo sp3 hybridization with bond angles 109.5о and bond lengths 154 pm. The whole
lattice is the continuous because of C — C covalent bonding.
 —
—  —
— 
Entire diamond crystal behaves as a huge giant three-dimensional carbon moleculel may also called macromolecule.
Why the lattice energy of large size cations and anions is less than that of smaller cations and anions?
The lattice energy of large size cations and anions is less than that of smaller cations and
anions due to less packing of ions.
Lattice energy is the energy which is released when one mole of ionic crystal is formed from its gaseous ions.
As the size of cations or anions increases, there is less packing of ions and so less energy is released during the formation.
Graphite is good conductor of electricity but diamond is bad conductor of electricity give reason.
Graphite is good conductor of electricity because one of the four valance electrons of
carbon atom is not covalently bonded with neighboring carbon atom that is this electron is free electron which helps to conduct electricity.
Diamond is an electrical insulator due to absence of free electrons in its crystal lattice because every carbon has utilized its four electrons in the formation of four covalent bonds. Therefore no single free electron is available for conduction of electric current in diamond crystal lattice.
Define molecular solids.
Those solids in which polar or non-polar molecules are held together by Vander Waal’s forces.
Types of molecular solids
1. Polar Molecular solids: The H2O molecules in ice.
2. Non-Polar Molecular solids: The molecules of I2(s).
What types of intermolecular forces are found in molecular solids.
- Dipole – dipole interactions or hydrogen bonding.
- Van der Waal’s forces or London forces
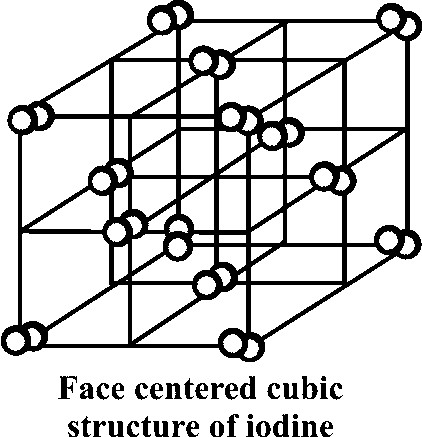
Write few lines on the structure of solid iodine.
- Molecules of iodine align in the form of layer lattice.
- Iodine is a poor conductor
- I-I bond distance = 271.5pm (in solid phase)
- I-I bond distance = 266.6pm (in gas phase)
Define metallic solid.
Those solids in which atoms of metals are held together by metallic bonds.
Metallic bond
Force which binds a metal cation to a number of electrons within its sphere of influence.
Write theories of metallic bonding.
1. Electron Gas Theory:
The theory proposed by Drude and extended by Loren in 1923.
Each atom in a metal crystal loses all of its valence electrons. These valence electrons form a pool or a gas. The cations are believed to be held together by electron pool or gas. This is called metallic bond.
2. Valence Bond Theory:
The theory proposed by L. Pauling.
Metallic bond is treated as covalent bond in character. The covalent bonds are not localized but are highly delocalized in metallic structure.
3. Molecular Orbital Theory (Band Theory):
The electrons in the completely filled orbitals are essentially localized, while atomic orbitals containing the valence electrons interact or overlap to form a set of delocalized orbitals.
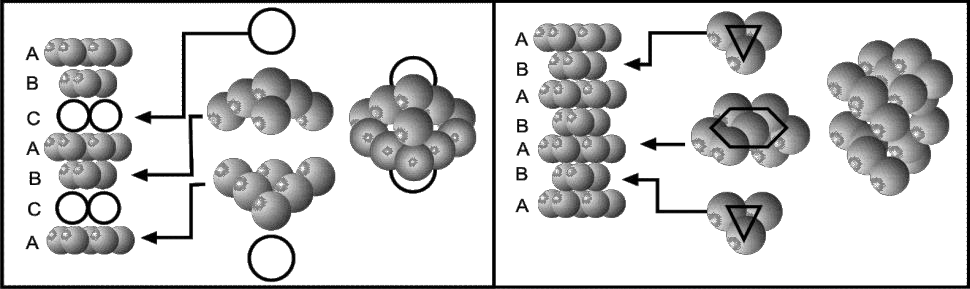
Describe packing of metallic structure.
- Cubic Close Packing (ABC, ABC or123,123)
- Hexagonal Close Packing (AB, AB or 12,12)
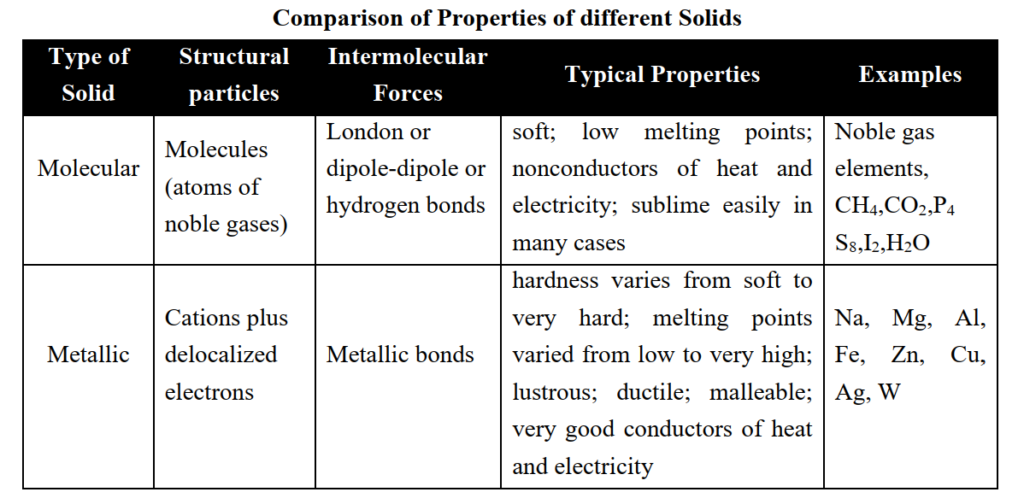
Comparison of Properties of different Solids
Write down steps to determine Avogadro’s Number (NA)
Calculate the followings.
- Density of one gram atom
- Volume
- Value of length
- Distance between the atoms
- Number of atoms along one edge length
- Ions