Umair Khan Academy provides FSc/MDCAT Students with effective notes to prepare for exams. ‘Alcohols Phenols and Ethers’ is an interesting chapter for class 12 students. Now it is under discussion. You can find other notes here. Or you can visit Umiar Khan Academy on YouTube for video lectures.
ALCOHOLS
Q1. Define Alcohols Phenols and Ethers in one definition.
They resemble with the structure of water, so we can say derivatives of Water molecules are called Alcohols Phenols and Ethers.
H-O-H (water), R-O-H (Alcohol), C6H5–O-H (Phenol), R-O-R (Ether)
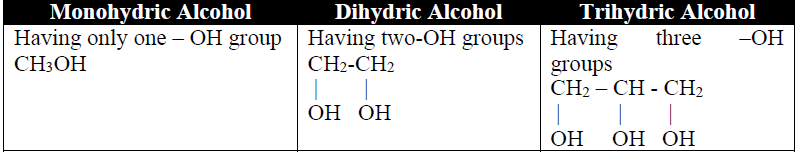
Q2. Define alcohols and write their classification.
The hydroxyl derivatives of hydrocarbons are called Alcohols.
1- Classification (on the basis of OH-group)
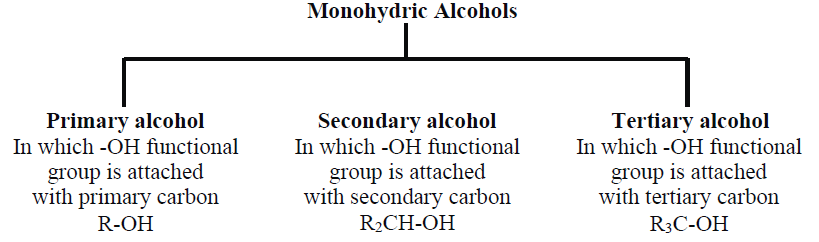
2- Classification of Monohydric Alcohols:
Depending on the nature of α-carbon, there are three types of monohydric alcohols.
NOMENCLATURE OF ALCOHOLS:
Q3. Write a few common/trivial names of alcohols.
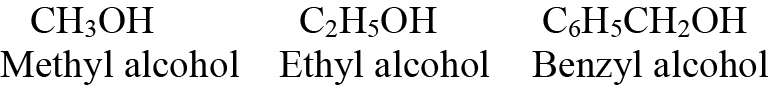
Q4. Write a few IUPAC names of Alcohols.
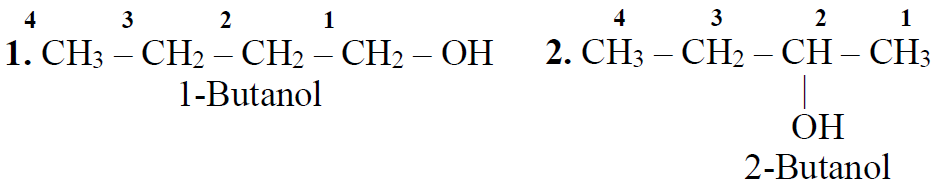
Preparation of Alcohol.
Q4. Write commercial preparation of Methanol.
From water gas:
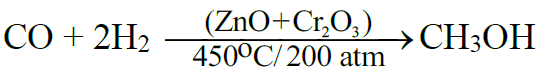
Q5. Write commercial preparation of Ethanol by fermentation.
Conversion of sugar into ethyl alcohol by yeast is called “alcoholic fermentation”.
From molasses:
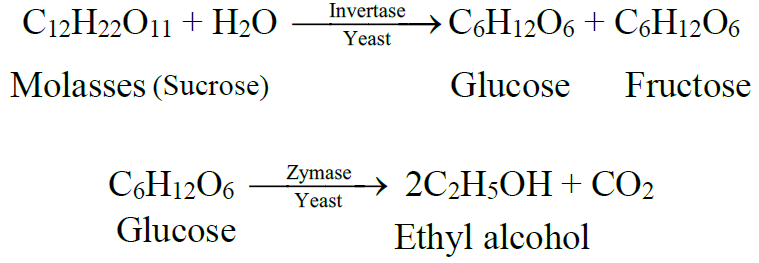
Q6. Define rectified spirit.
95% pure ethyl alcohol is called rectified spirit.
Q7. Define absolute Alcohol.
99.9% pure alcohol is called absolute alcohol.
Q8. What is Denaturation of Alcohols.
Ethanol is denatured by adding methanol, pyridine or acetone. In this way it becomes poisonous. If methanol is added, then the resulting mixture is termed as methylated spirit, which contains 10% methanol. This denaturing is purposefully done to avoid drinking.
PHYSICAL PROPERTIES
Q9. Write physical properties of alcohol.
- Colourless, toxic liquids, characteristic smell
- The boiling points of alcohols increase in regular way
- In isomeric alcohols, the boiling point decreases when branching increase.
- Melting and boiling points are much higher than those of corresponding alkanes.
- Lower alcohols are readily soluble in water but solubility decreases with rise in molar masses.
REACTIONS OF ALCOHOLS
Q10. Write the types of chemical reactions for alcohols and give examples.
Alcohols react with other reagents in two ways:
1- Cleavage of C—O bonds
Tertiary alcohol > Secondary alcohol > Primary alcohol
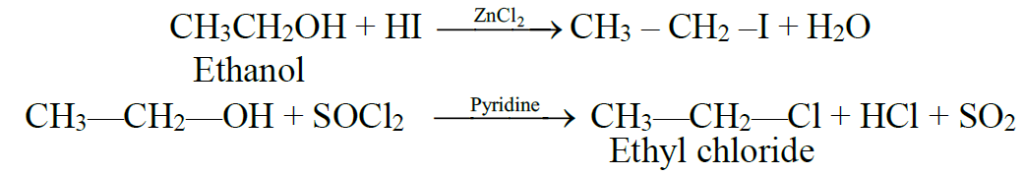
2- Cleavage of O—H bonds
CH3OH > Primary alcohol > Secondary alcohol > tertiary alcohol
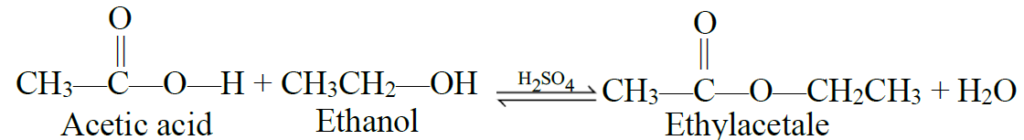
Q11. Write oxidation with alcohol.
1- Primary alcohol:
Primary alcohols give aldehydes on oxidation.
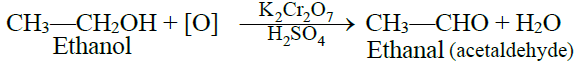
2- Secondary alcohol:
Secondary alcohols gives ketones on oxidation.
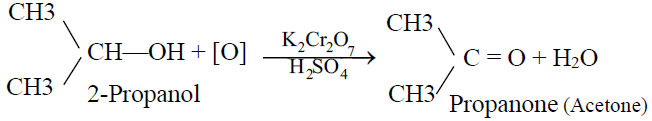
3- Tertiary alcohol:
Tertiary alcohol is resistant to oxidation and gives elimination product.
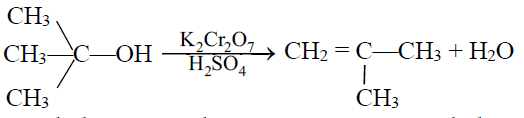
Q12. Write down the dehydration of ethyl alcohol.
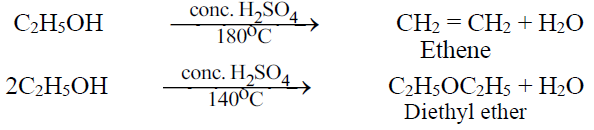
Q13. Write reactions of alcohol with Phosphorus Halides.
-
Reaction of alcohol with phosphorus trihalide:
3C2H5OH + PCl3 →3C2H5Cl + H3PO3 -
Reaction of alcohol with phosphorus trihalide:
C2H5OH + PCl5 →C2H5Cl + POCl3 + HCl
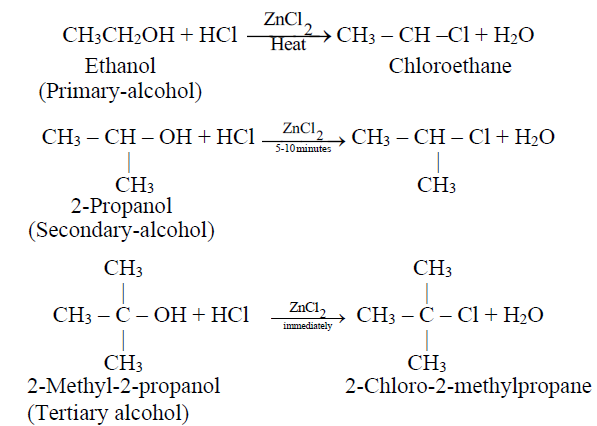
DISTINCTION BETWEEN PRIMARY, SECONDARY AND TERTIARY ALCOHOLS
Q14. Write down the distinction bewteen primary, secondary and tertiary alcohols.
By Lucas test:
Primary, secondary and tertiary alcohols are identified and distinguished by reacting them with concentrated HCl in anhydrous ZnCl2. An oily layer of alkyl halides separates out in these reactions.
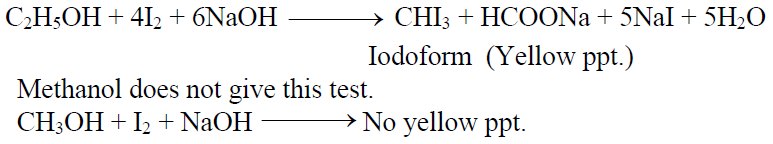
Q15. How to Differentiate between Methanol and Ethanol.
Ethanol gives iodoform test but methanol doesn’t.
Q16. Write down uses of alcohol.
- Uses of Methanol:
- as a solvent for fats, oils, paints and varnishes.
- as antifreeze in radiators of automobiles.
- for denaturing of alcohol as it is very poisonous.
- Uses of Ethanol:
- as a solvent, drink, fuel, preservative, in pharmaceutical
PHENOL
Q17. What is phenol?
The aromatic compounds in which —OH groups are directly attached with carbon atom of benzene ring.This is also called carbolic Acid.
PREPARATION OF PHENOL
Q18. Write down the methods of preparation of phenol.
1- Dow’s Method (from Chloro benzene)
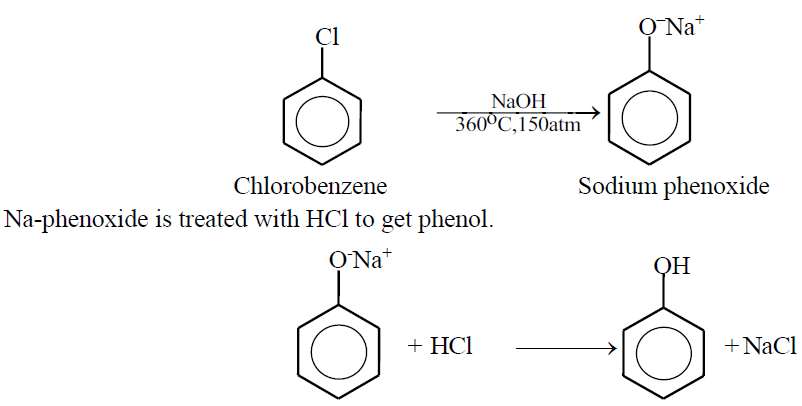
2- From sodium salt of benzene Sulphonic acid.
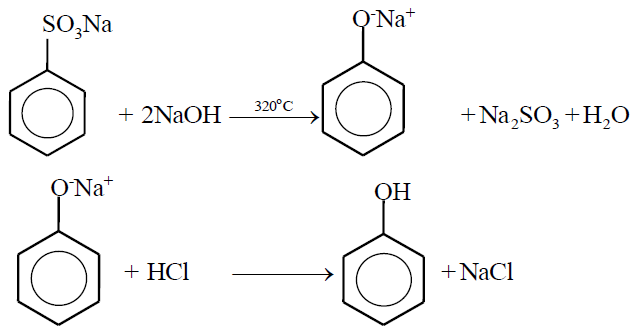
PROPERTIES OF PHENOL
Q19. Write dwon the physical properties of phenol.
- It is colourless, crystalline, deliquescent solid with characteristic smell.
- Its melting point is 41oC and boiling point 182oC.
- It is sparingly soluble in water forming pink solution at room temperature but completely soluble above 68.5oC.
- It is poisonous and used as a disinfectant in hospitals and washrooms.
Q20. Write down the chemical properties (Chemical reaction) of phenol.
A- Reactions due to —OH group:
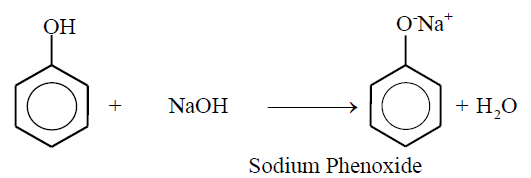
1- Salt formation:
Phenol reacts with alkalis to form salts e-g;
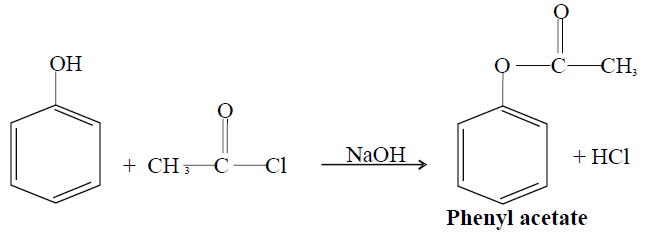
2- Ester formation:
Phenol reacts with acetyl chloride in the presence of a base to form ester.
3- Reduction with Zn:
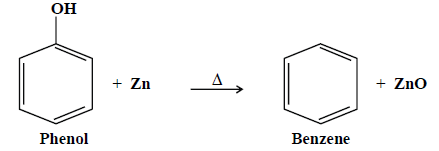
B- Reactions due to benzene ring:
1- Nitration
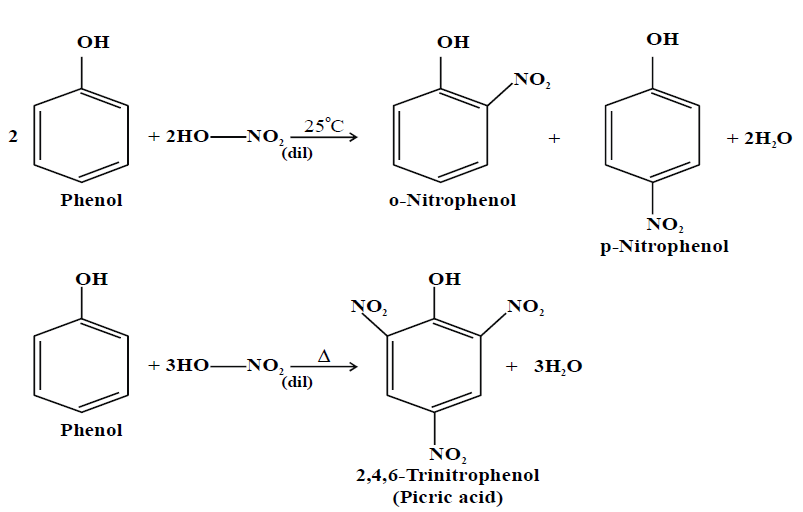
2- Sulphonation:
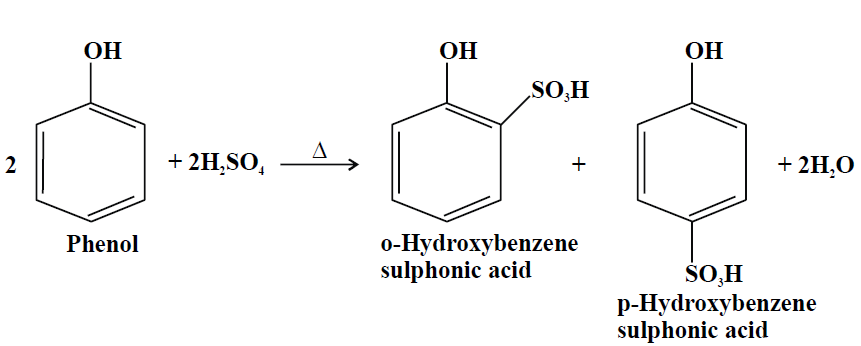
3- Halogenation:
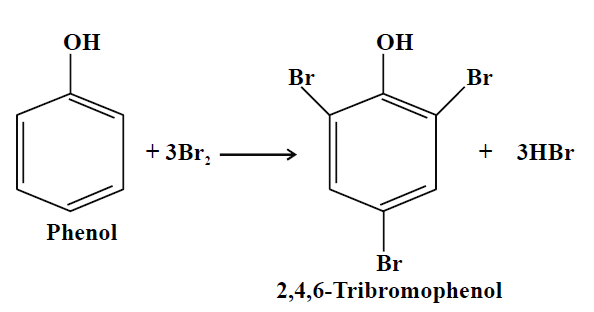
4- Hydrogenation:
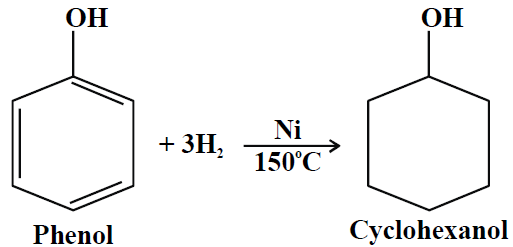
5- Reaction with formaldehyde:
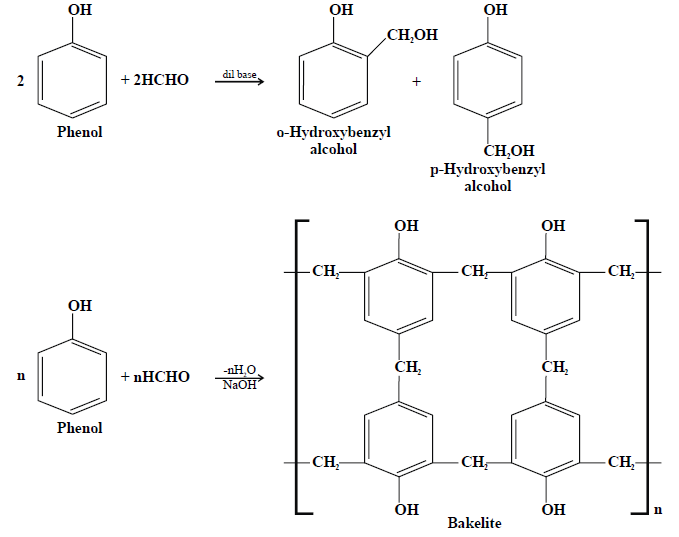
Ethers
Q21. What are ethers?
The compounds containing ether linkage (C-O-C) as a functional group are called ethers.
Q22. What is main classification of ethers.
NOMENCLATURE OF ETHERS
Q23. Write down methods of naming ethers, for common and IUPAC names.
Common names:
-
For symmetrical ethers:
While naming symmetrical ethers, prefix di is used with the name of alkyl group followed by the
word ether.
Example: CH3 – O – CH3 Dimethyl ether. -
For unsymmetrical ethers:
While naming the unsymmetrical ethers, alkyl groups are separately named in alphabetical order
followed by the suffix ether.
Example: CH3 – O – C2H5 Ethyl methyl ether.
IUPAC names:
-
For symmetrical ethers:
One side of ether is combined with oxygen atom to form alkoxy, and second side is assumed as a parent chain of carbon.
Example: CH3–O–CH3 Methoxy methane -
For unsymmetrical Ethers:
Smaller side is pronunced with oxygen and second side is written as parent chain of carbon.
Example: CH3–O–C2H5 Methoxy ethane
PREPARATION OF ETHERS
Q24. Write preparation of ethers.
1- Williamsons Synthesis
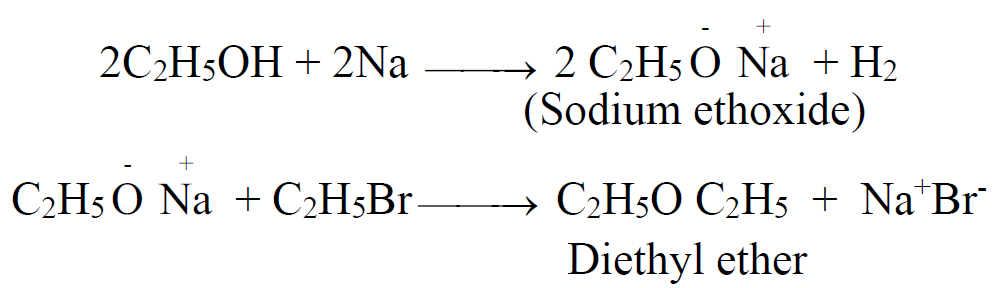
2- Reaction of alkyl halide with dry silver oxide Ag2O

PROPERTIES OF ETHERS
Q25. Write down the physical properties of ethers.
- Usually ethers are volatile liquids.
- They are highly inflammable with low boiling points.
- They are slightly soluble in water but freely soluble in organic solvents.
- They do not show hydrogen bonding with one another.
- They show weak hydrogen bonding with water so they are slightly soluble in water.
Q26. Write about chemical reactivity of ethers.
- They are comparatively inert compounds.
-
They are not attacked by reagents like ammonia, alkalis, dilute acids and
metallic sodium etc
in cold state. - They are not oxidized or reduced easily.
-
Reaction with hydrogen iodide.
Ethers react with HI to give alcohols through oxonium ion formation.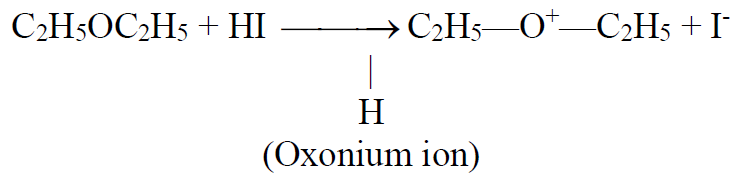
The iodide ion attacks on alkyl group to give alcohol and alkyl iodide.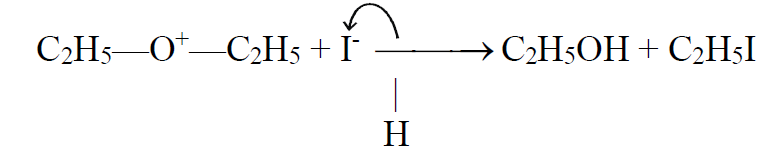
- Reaction with PCl5.
Ether react with hot PCl5 to give alkyl chloride.