The best method to prepare FSc part 1 chapter 4 Chemistry is through solving quizzes. And if you are worried about its preparation then the best notes are available.
Video Lectures are also available on my YouTube Channel (Umair Khan Academy)
Umair khan
Attempt MCQ quiz 1 FSc part 1 chapter 4 Chemistry(Liquids) Main Menu☝
Attempt MCQ quiz 2 FSc part 1 chapter 4 Chemistry(Liquids) Main Menu☝
Attempt MCQ quiz 1 FSc part 1 chapter 4 Chemistry(Solids) Main Menu☝
Notes for F.Sc. part 1 chapter 4 (Liquids) are in the form of very useful questions answers. Main Menu☝
Intermolecular forces
Q1. What is a dipole? Give examples.
It is a molecule that has two poles in it; these poles are created due to the difference in the electronegativities.
HCl has dipoles. H is partially positive while Cl is partially negative Similarly hydrogen bromide (HBr), hydrogen iodide (HI), carbon monoxide (CO), and NO etc. have dipoles in them.
Q2. What are dipole dipole forces?
Those forces of attraction, are present between the positive ends of one polar molecule and the negative ends of the other polar molecule. Molecule of water. attract each other due to the dipole-dipole attractions.
Q3. What is an induced dipole?
A molecule in which polarity is created due to the other molecule is called an induced dipole. Atoms of helium create dipoles in other atoms of He during their collision in the gaseous state.
Q4. What are Debye forces?
The forces of attraction that exist between already polar molecules and molecules having induced polarity are called dipole-induced dipole forces. These forces are also called Debye forces.
Q5. What is an instantaneous dipole?
The temporary dipole which is produced in non-polar atoms or non-polar molecules due to a certain reason is called an instantaneous dipole. Non-polar gaseous molecules create instantaneous dipoles. Helium is the best example. Other noble gases also have such dipoles.
Q6. What is a non-polar molecule?
A molecule in which there is no polarity. This is due to the absence of electronegativity difference between two bonded atoms. H2, O2, N2, Cl2 etc. are perfect nonpolar molecules. However, CH4 is non-polar due to the symmetry of its structure. CO2 is non-polar due to its linear structure.
Q7. What is a polar molecule?
The molecule which has a partial positive and negative charge on it due to the difference in electronegativity between the two bonded atoms is called a polar molecule.
HCl is a polar molecule. Similarly, HBr, HI CO, NO, H2O, H2S etc. are polar molecules.
Q8. What are dipole-induced dipole forces?
Those forces that are present among the molecule having a dipole and that molecule in which dipole moment has been induced are called dipole-induced dipole forces.
This induced dipole is due to the polar molecule which collides with the Molecule A is already polar after collision with B it makes it also polar.

Q9. What are ion-dipole forces? Give an example.
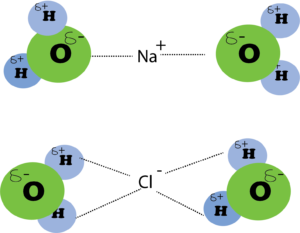
Those forces of attraction in which the negative end of the polar molecule are attracted towards the positive ion and the positive end of the polar molecule are attracted towards the negative ion. The NaCl dissolved in water creates ion-dipole forces. Na+ and Cl– ions are surrounded by water molecules as follows: the number of water molecules surrounding sodium ions and chloride ions depends upon the concentration of solution and sizes of ions. The forces of attraction between the ions in the water molecule are called ion-dipole forces.
Q10. Why do the melting and boiling points of halogens and noble gases increase down the group?
The atomic sizes increase from fluorine to iodine. The same is the case with noble gases. The number of shells increases down the group. So, polarizability increases and the tendency of overlapping of loosely held electrons also increases in both groups. This makes the melting and boiling point higher down the group.
Q11. Why dipole-dipole forces are much stronger than dipole-induced dipole forces.
In dipole-dipole forces, the atoms have sufficient partial positive and partial negative charges to attract each other. The example of forces in water, methanol and ethanol in individual vessels. In dipole-induced dipole forces, one of the atoms and molecules is nonpolar and the dipole has to be induced in that. This induced dipole is temporary and that is why the attraction between the dipole and induced dipole is comparatively weak.
Q12. What is polarizability? How it increases down the group in noble gases and is responsible for the increase in melting and boiling points of noble gases.
Polarizability is the capability of an atom, molecule or anion to be distorted for its electronic cloud. From the top to the bottom direction in the group, the atomic sizes increase in noble gases. Their outermost electronic clouds become a hue n away from the nuclei. These clouds are disturbed due to collisions. Temporary dipoles are developed and forces of attraction are created. For this reason, melting and boiling point increases down the group.
Q13. Why do the melting and boiling points of alkanes increase with the increase in molar masses and are less than that of the water?
Alkanes are saturated hydrocarbons such as CH4 C2H6 C3H8 C4H10 etc are gases. C5- C18 are liquids. They are all non-polar. Greater the length of the carbon chain, the greater the interaction of one molecule with another. Higher alkanes are zig-zag in structure and are called macromolecules as well. These features are responsible for the force of interactions and cause the increase of melting point and boiling point of alkanes. Water is a polar liquid and dipole-dipole forces are very strong.
Hydrogen Bonding
Q14. What is hydrogen bonding?
The electrostatic force of attraction between electronegative atoms like oxygen, nitrogen or chlorine etc, and partial positive hydrogens in some molecules is developed due to the hydrogen bonding. Ammonia, water, ethyl alcohol etc. have hydrogen bonding for the molecules in their pure.
Q15. Ice floats on water. Justify it.
Ice floats on water. water expands when it solidifies. This expansion is due to the empty spaces that are left behind due to the hydrogen bonding in the ice crystal. The density of ice is close to 0.91 g/cm 3 as compared to that of liquid water which is 1.00 gcm3 For this reason ice floats on water. no doubt, the density of water decreases above 4℃ but remains less than that of ice.
Q16. Water is a liquid at room temperature but H2S is a gas give a reason.
This is due to the high electronegativity of oxygen as compared to sulphur. water has hydrogen bonding due to the greater electronegativity difference of hydrogen and oxygen atoms. H2S does not have such bonding. Due to the absence of hydrogen bonding in H2S, It is a gas at room temperature.
Q17. Water freezes from the surface to the downward direction in ponds and lakes. Explain Why?
Ice has a lower density than liquid water at 0℃. It causes the water in ponds and lakes to freeze from the surface in a downward direction. Water retains the temperature of 4℃ by the fall of temperature in surroundings. As the outer atmosphere becomes colder, the water at the surface becomes less dense. This less dense water below 4℃ stays on top of the slightly warm water underneath. A stage reaches when it freezes. The layer of ice insulates water underneath for further heat loss. in this way, water at 4℃ maintains its temperature.
Q18. Water in ethanol can mix easily in all proportions. Why?
Both water and ethanol have OH groups, so they can do the hydrogen bonding extensively. This is why they can mix in all proportions.
Q19. How in a very cold winter do fish in garden ponds owe their lives to hydrogen bonding?
When water is frozen at 0℃ then it expands. The hydrogen bonding in the solid state of water adjusts the molecules of the water in such a way that empty spaces are left behind. In this way, the density of the war in the solid state becomes less. So it floats on water. In winter upper surface of the water in ponds can freeze. Ice being lighter comes to the surface. The water at 4℃ has maximum density and remains under ice, where animals can survive.
Q20. Give the reason for the low boiling point of hydrogen fluoride (19.5 ℃) as compared to the water (100℃). Although the hydrogen bonding in HF is stronger than that of H2O.
In water, each molecule can make two hydrogen bonds with two neighbouring water molecules. in this way, the hydrogen bonds in water molecules are greater. In the case of HF, there is only one partial positive hydrogen which can make only one hydrogen bond with another fluorine atom. So the greater the number of hydrogen bonds in water makes its boiling point higher. However the individual hydrogen bond in HF is indeed stronger than that of water due to the higher electronegativity difference.
Q21. How the cleansing action of soaps and detergents is possible due to the hydrogen bonding?
Soaps and detergents are the sodium salts of long-chain Carboxylic acids and benzene sulphonate respectively. The sodium acetate group (COO–Na+) of soaps and SO3–Na+ group Of detergents have a negative charge on oxygen atoms and create hydrogen bonding in water. The rest of the portion of the soaps and detergents remain outside water. In this way, water can do cleaning action and remove the dirt and greasy material from fabrics.
Evaporation and Vapour Pressure
Q22. Define evaporation. Why does it happen?
It is the spontaneous change of the liquid into vapour above its surface. It happens at all temperatures. The higher the temperature, the higher the rate of evaporation. The favourable collisions of liquid molecules at the surface of the liquid are responsible for this. The favourable collisions are those in which the energy of escaping molecules due to the collisions are greater than intermolecular forces.
Q23. What is vapour pressure?
The pressure which is exerted by the vapour of a liquid on the surface of the liquid at an equilibrium state, under the given conditions of the temperature is called vapour pressure. it depends upon the temperature and nature of the liquid. The vapour pressure value of the ethyl alcohol in ether at 20 ℃ is 43.9 torr and 442.2 torr respectively.
Q24. Why is the heat of vaporization of water is greater than methane (CH4)?
Water is a polar liquid and due to the strong hydrogen bonding, high energy is required to separate the molecules from each other at their boiling point. Methane is non-polar and has weak London dispersion forces.
Q25. How does the rate of evaporation depend upon the surface area?
A glass of water thrown on the floor evaporates in a short time but in glass, it may take months. Evaporation is a surface phenomenon. The greater the surface area greater the number of molecules present on the surface, and the greater the chances of water leaving the surface. This makes a greater evaporating tendency of the liquid.
Q26. The vapour pressure of liquids depends upon intermolecular forces, sizes of molecules and temperature of liquids. why do solids have low vapour pressure?
The greater the intermolecular forces, the lesser the evaporating tendency and the lesser the vapour pressure. when the molecules are big sized then evaporation tendency is less and vapour pressure is lower. Higher temperature increases the kinetic energy of the liquid molecules and the vapour pressure is also increased. The particles of the solid are tightly held with each other and the solid shows very low vapour pressure.
Q27. Evaporation of the liquid causes cooling. Why?
High energy molecules escape and change into vapour during evaporation. So, the temperature of a liquid falls. To compensate for that, heat flows from the surroundings to the region of the low temperature. This causes the temperature of the surroundings to decrease. so, evaporation causes cooling.
Q28. Evaporation takes place at all temperatures. How?
The molecules whose kinetic energies are greater than the average kinetic energy of the molecules, escape from the surface of the liquid. If the temperature of the liquid is increased, the rate of evaporation also increases. Anyhow, evaporation takes place at all temperatures and only the rate differs with the temperature.
Q29. Why do earthenware vessels keep water cool?
Earthenware vessels are porous. Water molecules come out from these pores and evaporate. These molecules of water need the energy to overcome intermolecular attractive forces. They get this energy from another molecule of water. Thus the temperature of the remaining water decreases.
Q30. One feels a sense of cooling under the fan after a bath.
When one takes a bath and sits in front of a fan, water on the surface of the body evaporates at a greater rate. The high-energy molecules escape from the surface of the body and one feels a sense of cooling. The reason is that the heat of the body is used up to evaporate water.
Q31. Why the vapour pressures of water, ethyl alcohol and diethyl ether are different from each other at 0℃?
Water, ethyl alcohol and diethyl ether have different extents of hydrogen bonding in their molecules. Water has maximum hydrogen bonding, ethyl alcohol has less hydrogen bonding and diethyl ether has no hydrogen bonding. For this reason, the vapour pressure of water at 0℃ is 4.529 torr, that of alcohol is about 15 torr and is 200 torr for diethyl Ether at 0℃.
Boiling Point
Q32. Define boiling point and give examples.
The temperature at which the vapour pressure of a liquid becomes equal to the external pressure is called the boiling point of the liquid. If the external pressure is 1 atm, then the boiling point is called the normal boiling point or just the boiling point. The boiling point of water is 100℃ and that of ethanol is 78.5℃ at one atm pressure.
Q33.Define the molar heat of fusion.
It is the amount of heat that is absorbed by one mole of a solid to melt it into liquid at its melting point, with the external pressure of 1 atm. The molar heat of the fusion of water is higher than that of methanol.
Q34. Define molar heat of sublimation.
It is the amount of heat that is required by one mole of a solid substance to convert it directly into one mole of its vapour at a particular temperature and one atmospheric pressure. Naphthalene can sublime at its heat of fusion is not that much. Similarly, I2 NH4Cl etc. can also sublime.
Q35. Define the molar heat of vaporization.
It is the amount of heat that is required to vaporize one mole of a liquid at its boiling point into its vapours. Water can vaporize and its molar heat of vaporization is sufficiently high. It is denoted by △Hv. For H2O, NH3, SO2 and Br2 these are + 40.6, + 21.7, + 24.3 and + 15.0 KJ/mol-1.
Q36.The boiling point of water is different at Murree Hills and Mount Everest. Justify it.
The boiling point of liquid changes as the external pressure changes. At Murree Hills, atmospheric pressure is less than the standard pressure of 760 torr. So, water boils at 98℃ instead of 100℃. At Mount Everest, atmospheric pressure is further reduced, so water boils at 69 ℃.
Q37. What are the advantages of vacuum distillation?
In vacuum distillation, the boiling point of a liquid is decreased by reducing the pressure by the suction pump.
- It reduces the time.
- Apparatus is saved from high temperature.
- The sensitive compound is not decomposed.
- Fuel is saved.
Q38. Vacuum distillation can be used to avoid the composition of a sensitive liquid.
The boiling points depend upon external pressure. In vacuum distillation, external pressure is reduced by vacuum. So, the boiling point of the liquid decreases. Hence sensitive liquids, like glycerine can be distilled safely without decomposition by vacuum distillation.
Q39. Define boiling point. Is it related to external pressure?
The boiling point is the temperature of the liquid at which the vapour pressure of the liquid is equal to the external pressure. If the external pressure is higher then the boiling point of the liquid is increased. If the external pressure is decreased, then the boiling point increases. The boiling point of water is lower at mountains than at sea level.
Q40.Boiling needs a constant supply of heat. Why?
When a liquid boils excess heat does not increase the temperature of the liquid. It is used to break the intermolecular forces of attraction among the molecules and convert liquid into vapours. The extra heat is absorbed by the molecule to become vapour. So boiling needs a constant supply of heat. Moreover, the molecules with more energy constantly leave the liquid.
Q41. Why things can be easily cooked in a pressure cooker?
If the water in the form of vapours is available at high temperature to the food particles then things can be cooked easily. In the pressure cooker, the external pressure is increased artificially, this thing increased the boiling point of the water. With the high boiling temperature as compared to 100℃, the water can cook things easily.
Sublimation
Q42. Why the heat of the sublimation of a substance is greater than that of the heat of vaporization? Give an example of Iodine.
During sublimation, two stages are crossed in a single step.
- Conversion of solid to liquid
- Conversion of liquid to vapours
In vaporization, liquid changes into vapour only and the energy is needed to perform one step. Therefore, the heat required for sublimation is greater than that of vaporization.
Liquid Crystals
Q43. What are liquid crystals?
It is a liquid turbid phase of a substance where there exist two things together, fluidity like liquid and orderly arrangement of particles like a crystal. This phase has both the properties of liquids and solids it has viscosity and surface tension while like crystals it has optical properties.
Q44. How liquid crystal can be used as a temperature sensor?
The liquid crystal can reflect light. When any of the wavelengths of light is reflected, the liquid crystal looks coloured. When the temperature is changed, the distance between layers of the molecules is changed. So the reflected light also changes colours. Due to this property of the cholesteric liquid crystals, they are used as temperature sensors.
Q45. State the biological applications of the liquid crystals.
Cholesteric liquid crystals are used to locate the affected parts of the body by applying them to the surface of the skin, comma, veins, arteries. They are also used to check breast cancer. The reason is that the affected parts are warmer than the surroundings and the liquid crystal changes its colour where it feels more temperature.