Carboxylic Acid is the last chapter in the Organic section, in Class 12 chemistry. Carboxylic Acid notes are given below. Material is specially designed to revise the whole chapter in a few minutes. Short questions and Extensive questions are in focus. For all notes, quizzes and helping material visit Umair Khan Academy.
Introduction to Carboxylic Acid
Q1. Define Carboxylic Acid.
The organic compound which contains —COOH group as a functional group is called Carboxylic Acid.
Q2. Write the Classification of Carboxylic acids depending upon the group R or Ar.
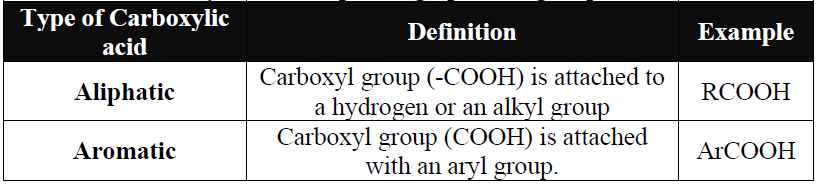
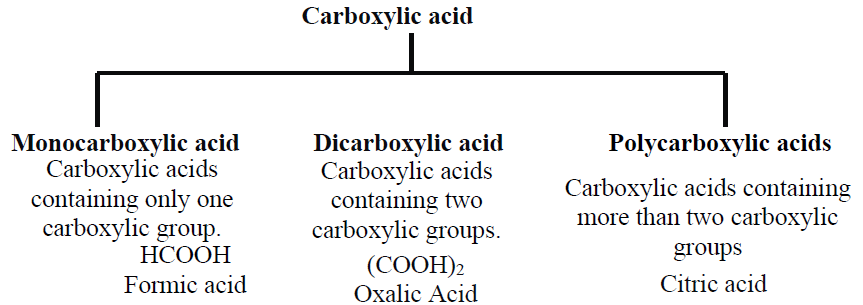
Q3. Write the Classification of Carboxylic acids depending on the Carboxyl group.
Q4. Write nomenclature for a few carboxylic acids.

GENERAL METHODS OF PREPARATION OF CARBOXYLIC ACID
Q5. Write down the general methods of preparation of carboxylic acid.
1- From Primary Alcohols and Aldehydes:
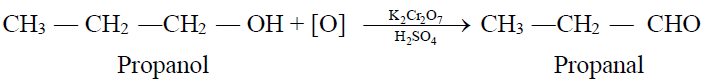
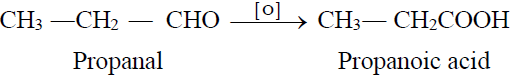
2- From Aldehydes:
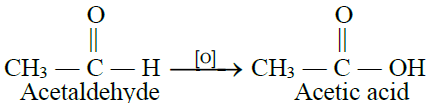
3- From Alkyl Nitriles:
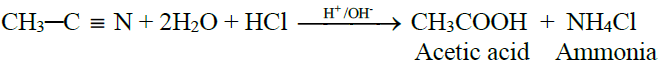
4- From Grignard Reagent:
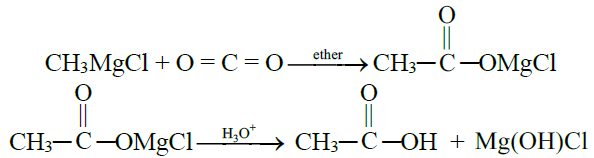
5- From Hydrolysis of Esters:
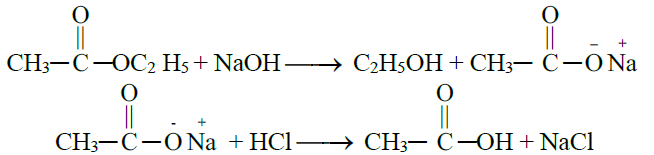
6- Commercial method / By the Oxidative Cleavage of Alkenes:
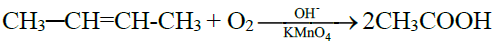
PROPERTIES OF CARBOXYLIC ACID
Q6. Write the physical properties of Carboxylic Acids.

Q7. Write down the reactivity of Carboxylic Acid.
There are three types of reactions given by carboxylic acids.
A. Reactions Involving Hydrogen atom of the Carboxyl Group:
Carboxylic acids are weaker acids than mineral acids. They furnish H+ when dissolved in
water
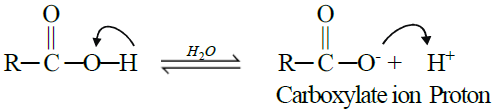
1- Reactions with Bases

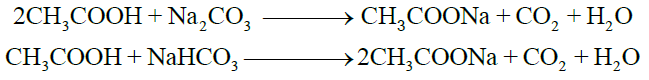
2- Reactions with Carbonates and Bicarbonates
3- Reactions with Metals

B- Reactions involving the –OH group of carboxylic acid:
1- Reaction with PCl3
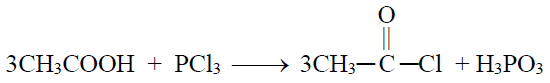
2- Reaction with PCl5
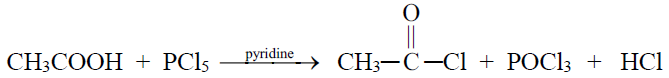
3- Reaction with SOCl2
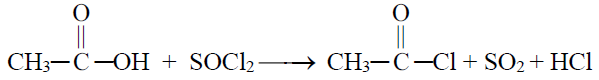
4- Formation of an ester (Esterification)
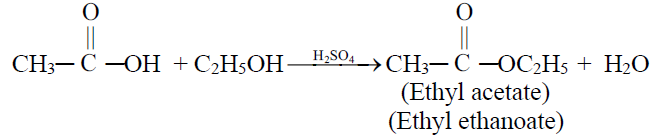
Examples of Some Esters
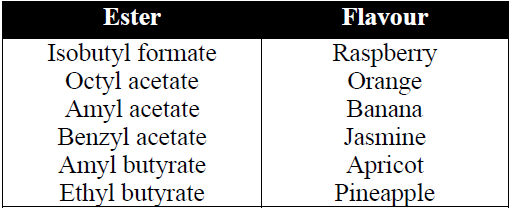
5- Reaction with NH3 (Formation of amide)
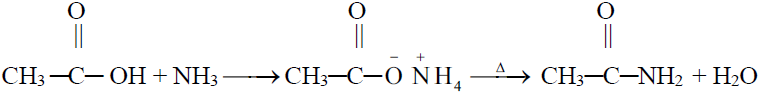
6- Formation of Acid anhydride (Dehydration)
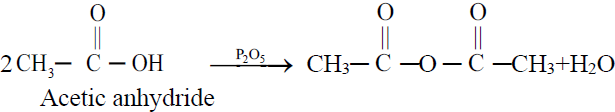
C. Reactions Involving Carboxyl Group (⎯ COOH)
1- Partial Hydrogenation (Reduction to alcohol).
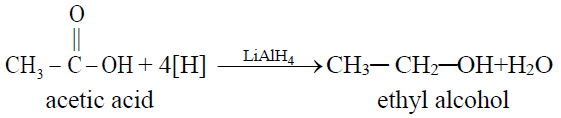
2- Complete Hydrogenation (Reduction to alkanes)
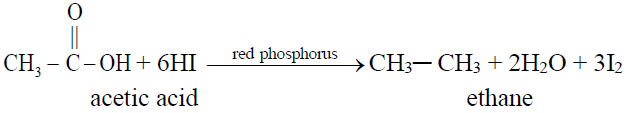
Q8. What are fatty acids? Give two examples.
The organic compounds which contain —COOH group as a functional group are called
carboxylic acids.
The aliphatic monocarboxylic acids are commonly called fatty acids because higher members of
this series such as palmitic acid (C15H31 -COOH), stearic acid (C17H35 COOH) etc. are obtained
by the hydrolysis of fats & oils.
ACETIC ACID
Q9. Define vinegar.
A dilute solution of acetic acid is called vinegar.
Q10. Write down the Laboratory Preparation of Acetic Acid or Ethanoic Acid.
1- By the oxidation of ethyl alcohol or acetaldehyde
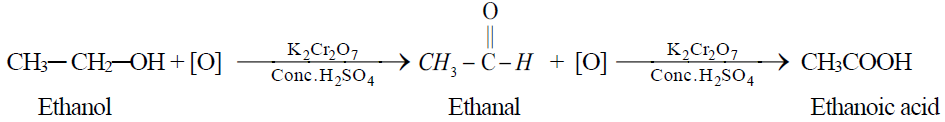
2- By the Hydrolysis of Ethanenitrile
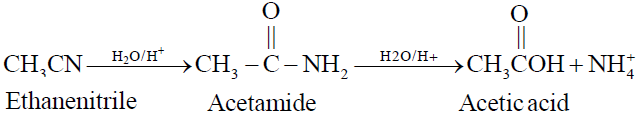
Q11. Write Manufacture of Acetic Acid on a large scale. (Industrial Preparation)
1- From acetylene:
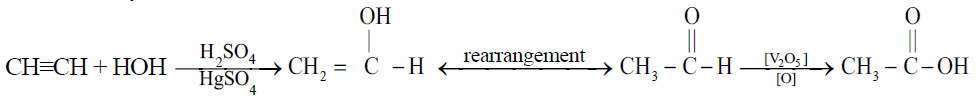
2- By Fermentation
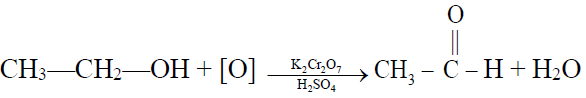
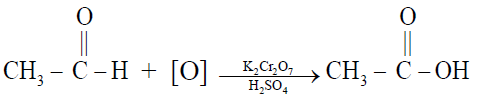
Q12. Write down the Physical characteristics of Acetic acid.
- Acetic acid is a colourless liquid with a boiling point of 118oC.
- It has a strong vinegar odour and sour taste.
- The pure acid freezes to an ice-like solid at 17oC. Therefore, it is also called glacial acetic
acid. - It is miscible with water, alcohol and ether in all proportions.
- Reactions of acetic acid:
Chemical reactions of acetic acid are similar to general reactions of carboxylic acids.
Q13. Write USES of Acetic Acid.
- As a coagulant for latex in the rubber industry.
- In the manufacture of plastics, polyvinyl acetate, rayon (cellulose acetate) and silk.
- In medicine as a local irritant.
- As a solvent in the laboratory for carrying out reactions.
- In the manufacture of pickles.
- In the manufacture of many organic compounds like acetone, acetates and esters.
AMINO ACIDS
Q14. What are amino acids?
The compounds containing an amino group and carboxylic acid group together are called amino acids. It has the following general formula.
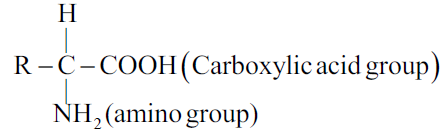
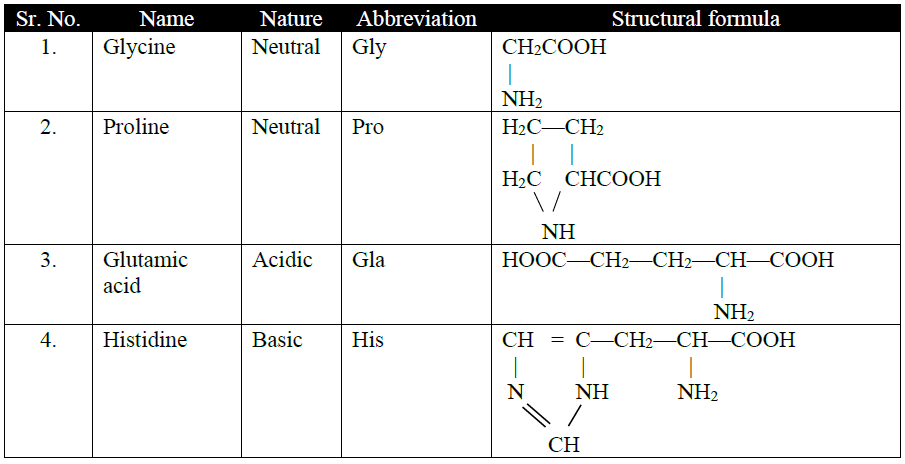
Q15. Write the classification of Amino Acids.
(a) On basis of attachment of amino group:
1- α -amino acids
2. β -amino acids
3. γ -amino acids
Naturally occurring amino acids are α-amino acids.
(b) On the basis of synthesis in the body need
1- Essential: The amino acids which our body cannot synthesize
2- Non-essential: The amino acids which our body can synthesize
(c) Number of NH2 or COOH groups:
1- Acidic amino acids
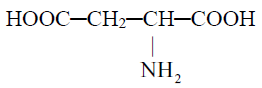
2- Basic amino acids: Contain two amino group
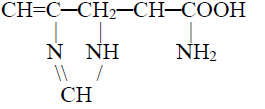
3- Neutral amino acid: Contains one amino and one carboxylic acid group
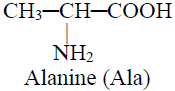
Q16. Write Nomenclature of Carboxylic acid.
IUPAC names are not extended because they are not easy to remember as they are lengthy.
Trivial names depend upon
- Origin
Example: Tryosine i.e. Tryos – Cheese - Obvious properties
Example: Glycine i.e. Glykys – sweet
Q17. What is Zwitter ion? Write some information.
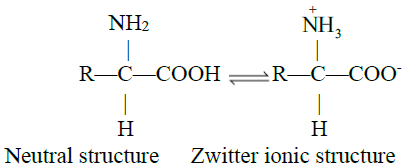
Acidic and Basic character of Amino Acids:
- When acid is added to amino acid, the carboxylate ion accepts the proton
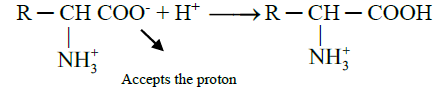
- When an alkali is added to an amino acid, the NH3+ group releases the proton
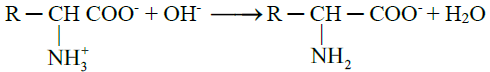
Q18. Write synthesis of amino acid.
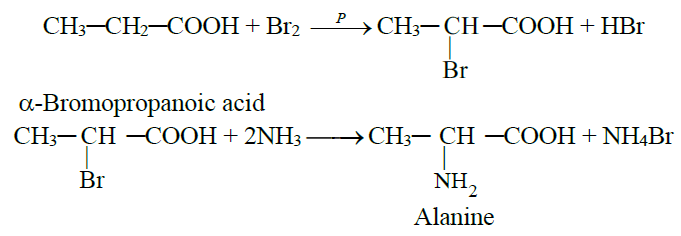
Following are the two methods for the synthesis of amino acids.
1- By the reaction of α-bromoacid with ammonia
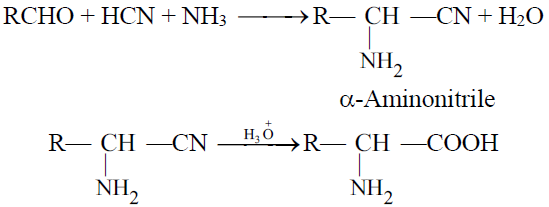
2- The Strecker’s Synthesis:
Q19. Write important chemical reactions of Amino Acids.
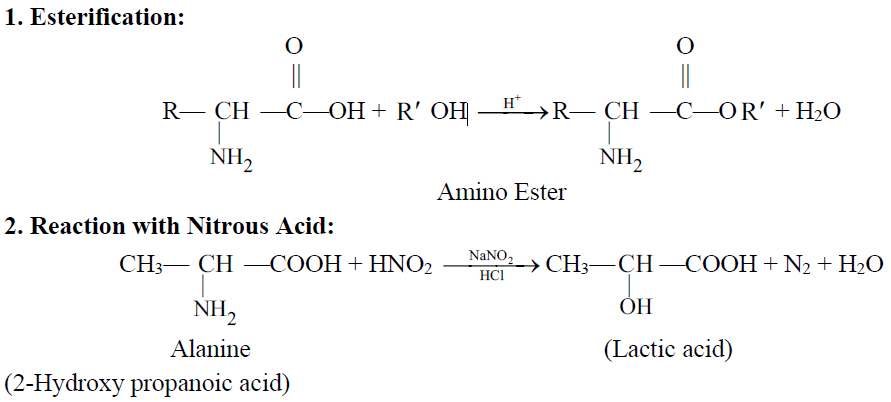
Q20 How to test an amino acid.
Ninhydrin Test
Ninhydrin is used as a locating agent and the spot of amino acid becomes intensely bluish-violet in colour.
Q20. What is a peptide bond? Write down the formula of a dipeptide.
In peptide bond formation carboxyl group of one amino acid and the amino group of another amino acid get condensed with the elimination of water. The resulting -CO-NH linkage is called peptide linkage.
Formation:
The condensation occurs between amino acids with the elimination of water molecules. The resulting —CO—NH— linkage is called a peptide linkage. The formation of a peptide is shown below:
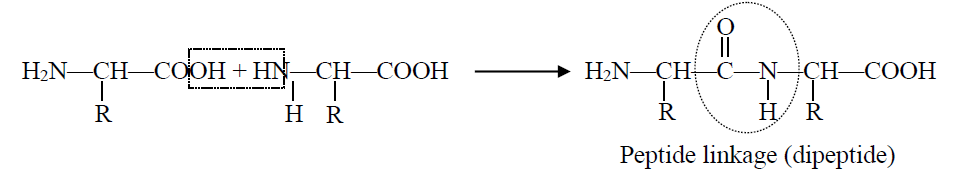
GET IN TOUCH
Visit YouTube Channel