Chemistry notes on the chapter Atomic Structure are included on this page. Students can take advantage of exam preparation, as the whole chapter is summarized in terms of short questions. A few short questions could be combined to make an extensive question.
Atomic Structure
Q. Write a few examples of Sub-atomic Particles.
Example: Electron, proton, neutron, neutrino, positron, etc
DISCOVERY OF ELECTRONS (CATHODE RAYS)
Q. What is a Discharge tube?
A glass tube filled with a gas fitted with two electrodes, a cathode and an anode is called a discharge tube.
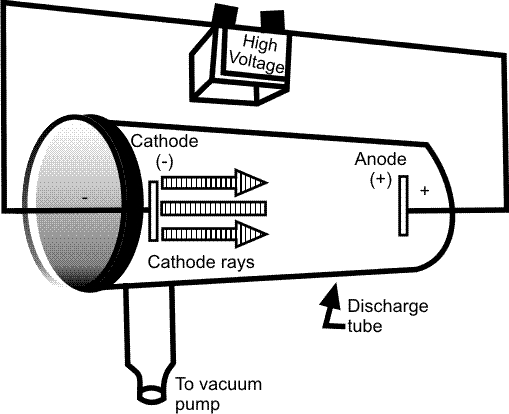
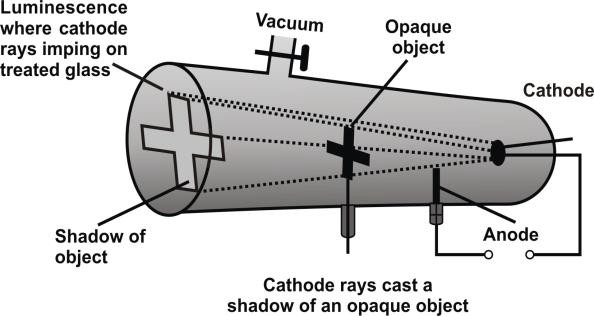
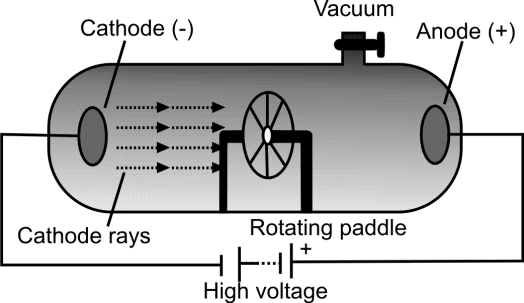
Q. Write down the PROPERTIES OF CATHODE RAYS
Cathode Rays:
- Are negatively charged
- Produce fluorescence Example: Alumina glows red and tin glows yellow.
- Cast shadow
- Can drive a small paddle wheel
- Can produce X-rays when they are converged through a concave surface on an anode of a large atomic number.
- Produce heat
- Platinum foil glows when cathode rays are focused on it from the concave cathode
- Can ionize the gas
- Can cause chemical change because they have a reducing effect.
- Can pass through the thin metal foil
- Same e/m of cathode rays
- Low penetration effect
Discovery of Proton (Positive Rays)
Q. Write down the reason for the production of the protons.
Reason for production
When high-speed cathode rays strike the residual gas molecules, they knock out electrons from
them and positive ions are produced.
M + 1e- → M+ + 2e-
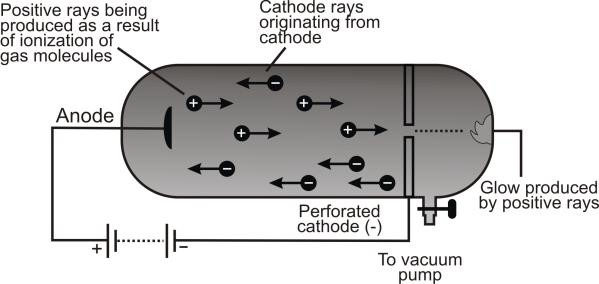
Observation:
These rays after passing through the perforated cathode produce a glow on the opposite to the anode.
Q. Write down the PROPERTIES OF POSITIVE RAYS.
- These rays travel in a straight line towards the cathode.
- The rays are positively charged.
- They cause a flash upon the ZnS plate.
- Their e/m ratio varies with the residual gas.
- Their e/m ratio is maximum in the case of hydrogen and smaller than cathode rays.
- These rays consist of tiny particles called protons (when hydrogen gas is used) having a mass 1836 times of electrons.
DISCOVERY OF NEUTRON
Q. How did the discovery of neutrons take place?
Procedure:
A stream of α-particles produced from a polonium source was directed at the beryllium target.
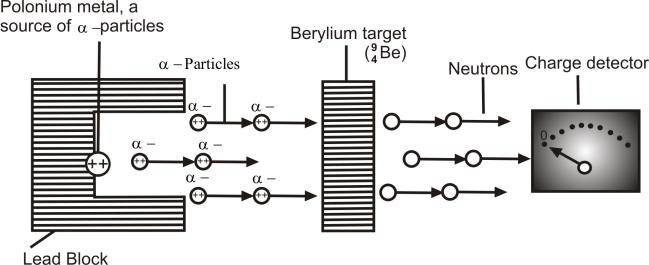
Observation:
Some penetrating radiations were produced. These radiations were called neutrons because the charge detector showed that they are neutral. 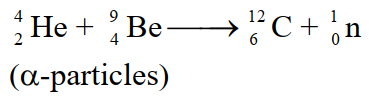
Actually α-particles and Be nuclei are re-arranged and an extra neutron is emitted.
Q. Write down the Properties of Neutrons.
- A free neutron decays into a proton
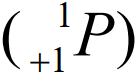 with the emission of an electron
with the emission of an electron 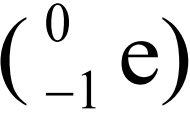 and neutrino
and neutrino 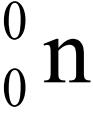 .
.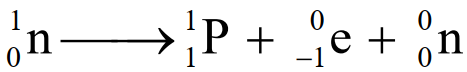
- Neutrons cannot ionize gases
- Neutrons are highly penetrating particles.
- They can expel high-speed protons from paraffin, paper, water and cellulose.
- Neutrons are of two types
(i) Fast neutrons travel with energy 1.2 MeV.
(ii) Slow neutrons travel with energy < 1 eV. - Slow neutrons are more effective than fast ones for fission purposes.
- A fast neutron ejects an α -particle and Boron is produced.
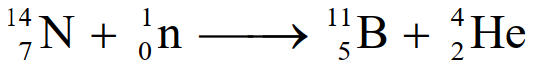
- Slow neutrons eject γ-rays when they hit Cu. Radioactive 65Cu29 is converted into 66Zn30.
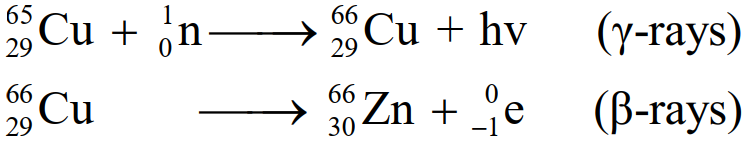
- Because of their intense biological effects, they are being used in cancer treatment.
Q. Why positive rays are called canal rays? OR
What are the canal rays and how are they produced in the discharge tube?
The positively charged rays produced in the discharge tube are called canal rays because
they pass through the porous cathode. The high energy electrons ionize the atoms of the gas
present in the discharge tube and make positive rays as,
M + e- → M+ + 2e-
Q. Why it is necessary to decrease the pressure in the discharge tube to get the cathode
rays?
This is because at high pressure the gas molecules hinder the movement of electrons between the electrodes. So the pressure is reduced to 0.01 torr, which allows the emission of cathode rays from the surface of the cathode.
MEASUREMENT OF e/m VALUE OF ELECTRON
Q. How to measure the e/m value of electrons.
Apparatus:
The apparatus consists of a discharge tube as shown below:
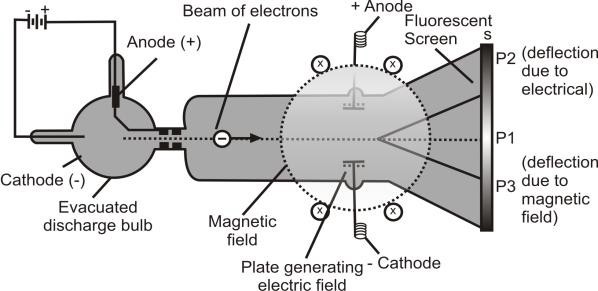
e/m Value:
By comparing the strengths of the two fields, the e/m value of electrons can be determined. It is 1.7588 × 1011 C kg-1. This shows that 1kg of electrons carry 1.7588 × 1011 coulombs of charge.
MEASUREMENT OF CHARGE ON ELECTRON BY MILLIKAN’S OIL DROP METHOD
- Millikan’s Apparatus:
The apparatus consists of a metallic chamber. - Atomizer:
A fine spray of oil droplets is created by an atomizer. - Illumination of droplet:
Droplet is illuminated which appears as a bright speck against a dark background. - Ionization of air:
The droplet takes up an electron and gets charged. - The velocity of droplet:
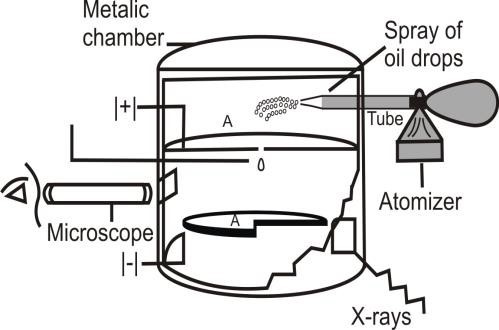
- Downward velocity of droplet:
The velocity of the droplet for downward movement depends upon its weight:
v1 ∝ mg —————— (I) - The upward velocity of droplet:
When the battery is switched on, the droplet moves upwards with a velocity of v2.
v2 ∝ Ee – mg ————– (ii)
Dividing (i) by (ii)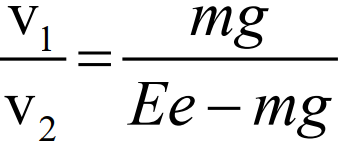
Q. What is the charge of an electron?
Millikan determined the charge on the electron to be 1.59 × 10-19 C. which is very close to the recent value
1.6022 × 10-19 C.
Q. What is the mass of an electron?
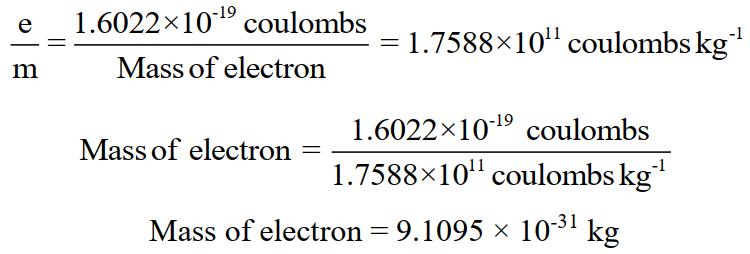
Q. Write a summary of the properties of Fundamental Particles.
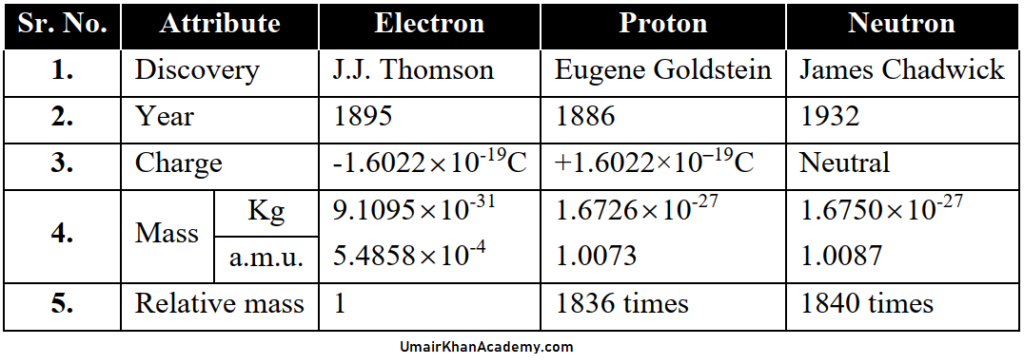
Q. Write down the RUTHERFORD’S MODEL OF ATOM
OR Write the DISCOVERY OF NUCLEUS.
Observations:
- Whenever an α-particle struck the screen, a flash was produced.
- Most of the particles went through foil un-deflected showing that maximum empty space.
- Some were deflected at fairly larger angles showing that there is a positive particle in the centre of an atom.
- A few were deflected backwards showing that there is a solid core in the centre.
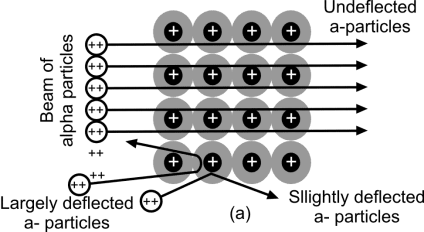
Conclusion:
Rutherford proved that the rebounding particles must have collided with the central heavy portion of the atom which was called a nucleus.
Q. Write down the defects of Rutherford’s atomic model.
- Rutherford’s model is based on the laws of gravitation and motion which are for neutral bodies.
- The moving electron should emit radiations continuously and so fall into the nucleus by classical theory. But it never happens.
- A revolving electron should emit energy continuously and a continuous spectrum but there is a line spectrum.
Q. WRITE PLANCK’S QUANTUM THEORY
Postulates of theory:
Energy is emitted or absorbed in a discontinuous manner in the form of energy packets.
- The energy of quanta is directly proportional to the frequency (ν) of radiation.
E ∝ ν
E = hν
h = E/ν - A body emits or absorbs energy in the form of a quantum of energy (hν).
E = hν
E = hc × 1/λ - The number of waves per unit length is called wave number(
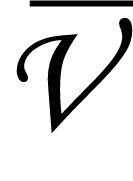 ). It is the reciprocal of a wavelength.
). It is the reciprocal of a wavelength.
If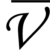 =1/λ
=1/λ
E = hc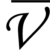
Conclusion of theory
a) E ∝ ν
b) E ∝ ![]()
c) E ∝ 1/λ
BOHR’S ATOMIC MODEL
Postulates:
- Electron revolves around the nucleus in circular orbits.
- The energy of an electron is fixed in one orbit.
- The energy of electron changes by ΔE when an electron jumps from one orbit to another orbit.
ΔE = E2 – E1
ΔE = hν - Electrons have fixed angular momentum
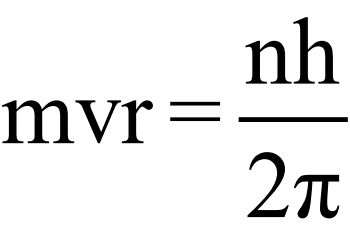
Where n = 1,2,3… and n = shell number
Q. What is Bohar’s radius?
r = 0.529(n2)Ao
Q. What is ENERGY OF REVOLVING ELECTRON
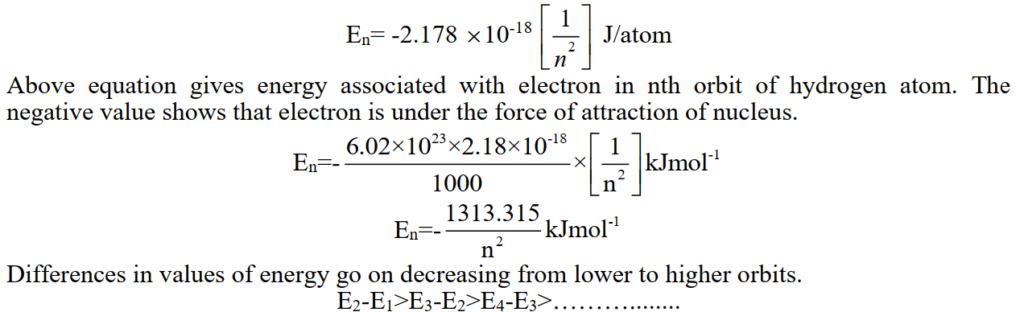
Q. Do you think that the size of Li+2 is smaller than He+? Justify with calculations.
Li+2 and He+1 both are mono-electronic systems but Li+2 has three protons and He+1 has two protons in the nucleus. According to Bohr’s model, the first radius of both ions is given by
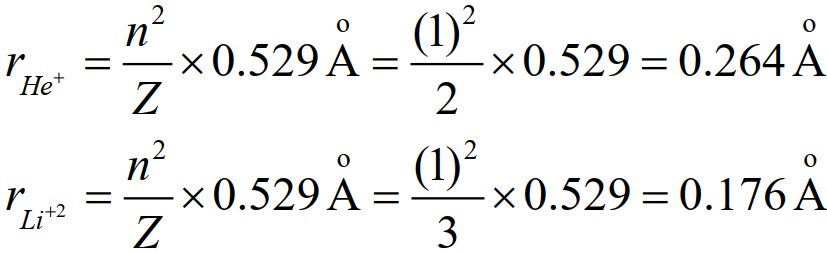
So the size of the first orbit of Li+2 is smaller He+1.
Q. Write two defects of Bohr’s atomic model.
1- Origin of the spectrum:
- Bohr’s theory can only successfully explain the origin of the spectrum in mono-electron (one electron system) system like H, He+,Li2+, Be+3 etc
- Bohr’s theory failed to successfully explain the origin of the spectrum of multi-electron or polyelectron system like He, Li and Be.
2- Fine structure or multiple structures of spectral line
The splitting of spectral lines into component lines while passing through a high-power resolving spectrometer is called multiple or fine structures. Bohr failed to explain the multiple structures of spectral lines.
Example: The Hα-line in the Balmer series is found to consist of 5-component lines.
3- Flat atomic model
Bohr suggested circular orbits of electrons around the nucleus which is a flat model of atom.
4- Doesn’t Explain Zeeman’s effect and Stark effect.
Bohr does not explain Zeeman’s effect and stark effect
Zeeman Effect
Splitting of a spectral line into a number of closely spaced lines in the presence of a magnetic field.
Stark’s Effect
Splitting of a spectral line into a number of closely spaced lines in the presence of an electric field.
Q. What is Sommerfeld’s modification of the atomic model?
Sommerfeld suggested that moving electrons describe elliptical orbits. In these orbits, the nucleus lies
in one of foci of an ellipse.
What is SPECTRUM
Definition:
The visual display or dispersion of components of white light, when it is passed through a prism, is called a Spectrum.
Example: Dispersion of light while passing through the prism.
Types of Spectrum:
Types of Atomic spectrum:
There are two types of atomic spectrums.
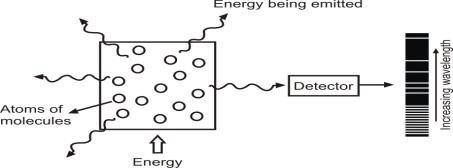
1. Atomic emission spectrum:
The spectrum of light obtained from the heated element appears as bright lines against a dark background.
Example: Spectrum of heated Hydrogen gas.
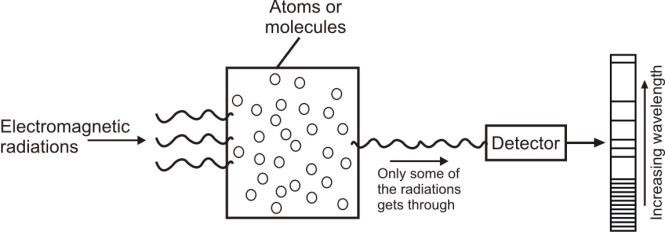
2. Atomic absorption spectrum:
It appears as dark lines against the bright background.
Example: Spectrum of white light, after being absorbed by Hydrogen gas.
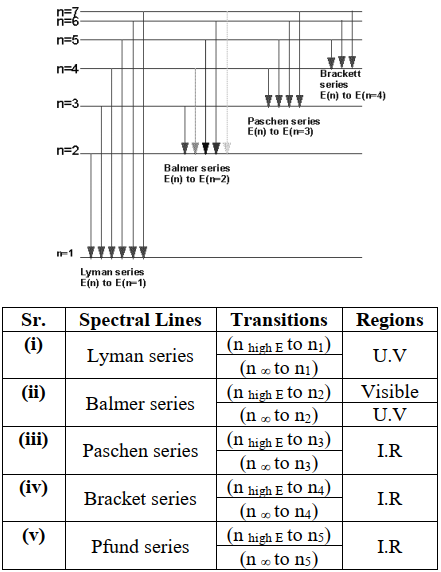
HYDROGEN SPECTRUM
Absorption of radiations:
Electron in hydrogen atom may revolve in any orbit depending upon its energy.
Emission of radiations:
When an electron in a higher orbit comes back to a lower orbit, the same energy is released
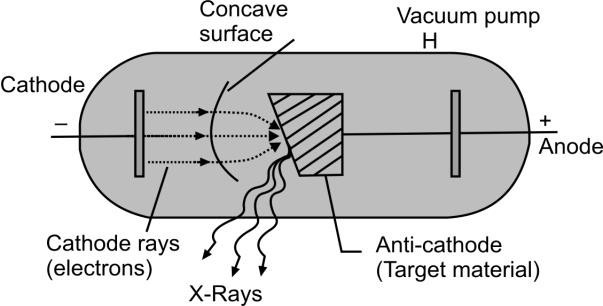
Q. What are X-RAYS and write its relationship with ATOMIC NUMBER?
X-rays are produced when rapidly moving electrons (cathode rays) collide with a heavy metal anode (anti-Cathode) in the discharge tube. Actually, X-rays are produced due to the inner shell transition of electrons.
- Moseley’s Law:
The frequency of X-rays is directly proportional to the square of an atomic number of metal emitting it.
√v = a(Z-b)
Where ‘a’ is a proportionality constant and ‘b’ is called the screening constant. - Production of X-Rays:
A high-energy beam of electrons.
X-Rays Spectrum:
X-rays are passed through a slit plate and thrown on a crystal of K4[Fe(CN)6].
Q. What was the Conclusion of Moseley’s Experiment:
- He classified spectral series into two distinct groups.
a) X-rays of shorter wavelengths are grouped as K-Series.
b) X-rays of longer wavelengths are grouped as L-series. - X-rays of shorter wavelengths are emitted when the target metal is of a higher atomic number.
Q. Write down the importance of Moseley’s Law
- Moseley arranged the elements K and Ar, Ni and Co in a proper way in Mendeleev’s
periodic table. - This law has led to the discovery of many new elements like Tc(43), Pr(59), and Rh(45).
- The atomic number of rare earths has been determined by this law.
Q. Throw light on WAVE-PARTICLE NATURE OF MATTER OR DUAL NATURE OF MATTER.
According to de-Broglie, all matter particles in motion have a dual character that is every material particle is also associated with wave-like properties. The de-Broglie equation for the dual nature of matter is as follows:
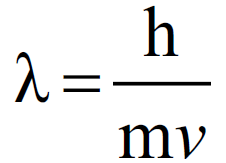
Applying de-Broglie’s Equation on Different Material Particles:
(i) Electron:
λ = 0.33 x10-9 m or 0.33nm
(ii) 1g Stone
λ = 6.626 x10-30m or 6.626 x10-21nm
Conclusion:
(i) This concept is only applicable to microscopic bodies.
(ii) This concept is not applicable to macroscopic bodies.
Q. Who verified the wave nature of moving electrons by experiment?
Davisson and Germer proved that accelerated electrons undergo diffraction just like waves when they fall on nickel crystal. In this way, the wave nature of moving electrons is verified.
Q. Define HEISENBERG’S UNCERTAINTY PRINCIPLE.
It is impossible to measure the position as well as the momentum of electron simultaneously and accurately.
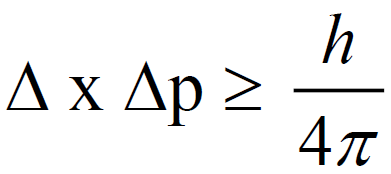
This principle is only applicable to microscopic particles whereas it is not applicable to macroscopic particles.
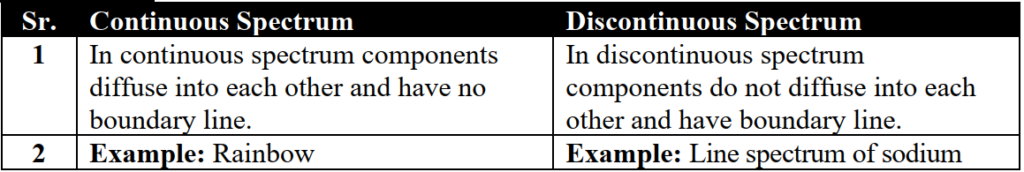
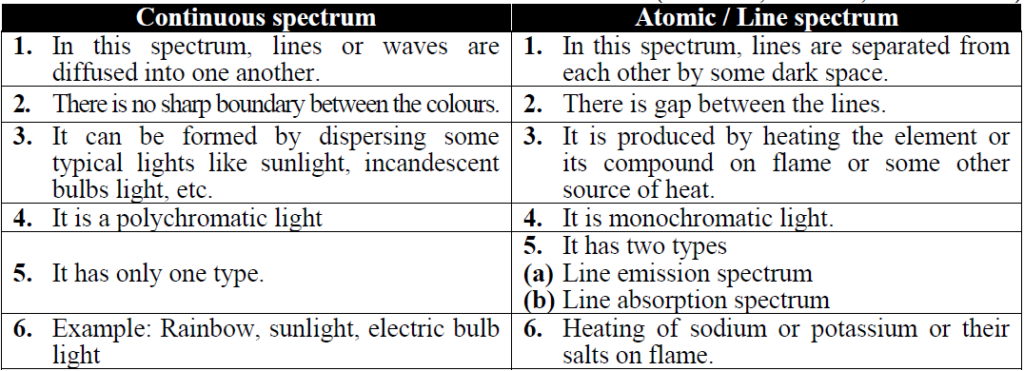
Q. What is the difference between continuous and atomic or line spectrum?
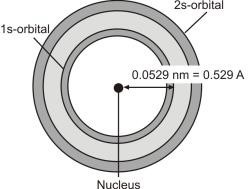
Q. Define Orbital.
The volume of space in which there is a 95% chance of finding an electron around the nucleus is called orbital. The maximum probability of finding the electron is at a distance of 0.0529nm.
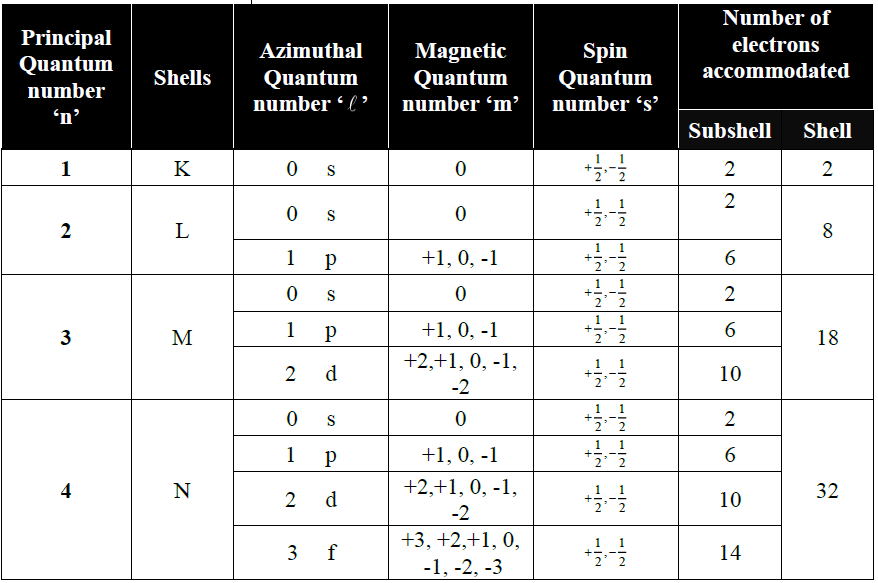
Q. What are quantum numbers? Write their types.
A set of numerical values which gives the acceptable solutions to Schrodinger
wave equation for the Hydrogen atom.
Types of quantum numbers.
1-Principal quantum number (n):
It gives us a quantitative measure of the size of the electronic shell. It also tells us the energy of a shell. It
is represented by ‘n’ and its values are non-zero, positive integers up to infinity. n=1, 2,3,4,5
where n represent the shell number.
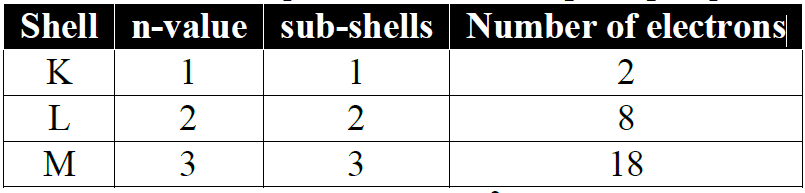
Applications of Principal quantum number:
- a) The number of sub-shells in a shell is equal to its ‘n’ value (principal quantum number).
- b) The no. of orbitals in a shell can be calculated by the ‘n2’ formula:
- c) The no. of electrons in a shell can be calculated by the formula 2n2.
2- Azimuthal quantum number (l ):
It gives us the idea of a subshell and the shape of subshell.
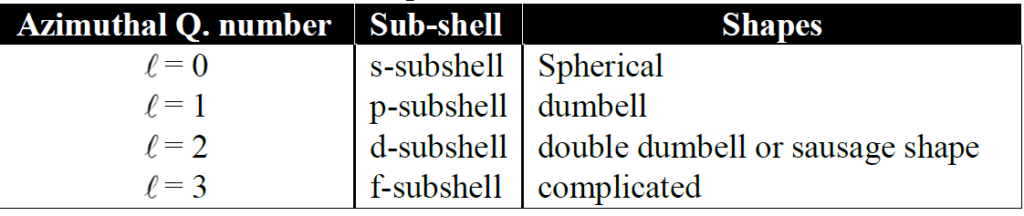
Applications of Azimuthal quantum number:
(a) Number of orbitals in a sub-shell is calculated by the formula (2l +1)
b) Number of electrons in a sub-shell is calculated by formula 2(2l +1)
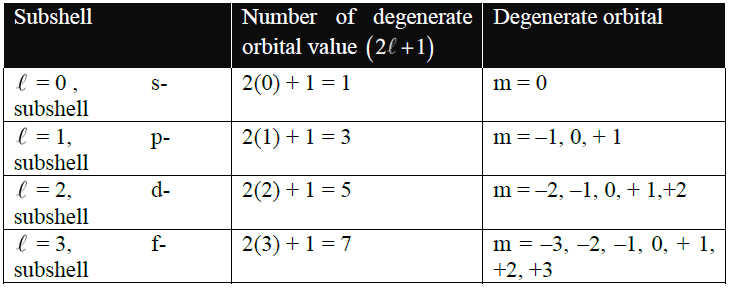
3- Magnetic or Orbital Orientation Quantum Number (m):
It gives us the idea of the number of degenerate orbitals present in a subshell.
The number of ‘m’ values can be calculated by the formula (2l +1).
4- Spin Quantum Number (s):
Clockwise and anti-clockwise spin of electron produces opposite magnetic fields. This spin motion of electrons is responsible for doublet line structure in the spectrum of alkali metals.
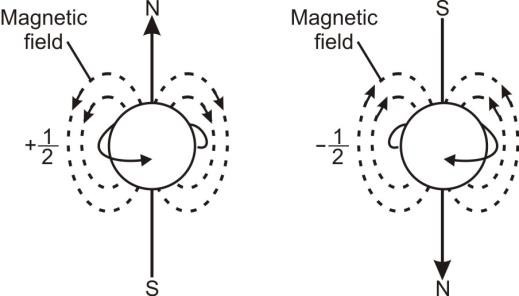
When the outermost excited electron of alkali metals jumps back to the ground state, each emitted line
seems to consist of a pair of lines when observed in high power resolving spectrometer. This is
called a doublet line structure.
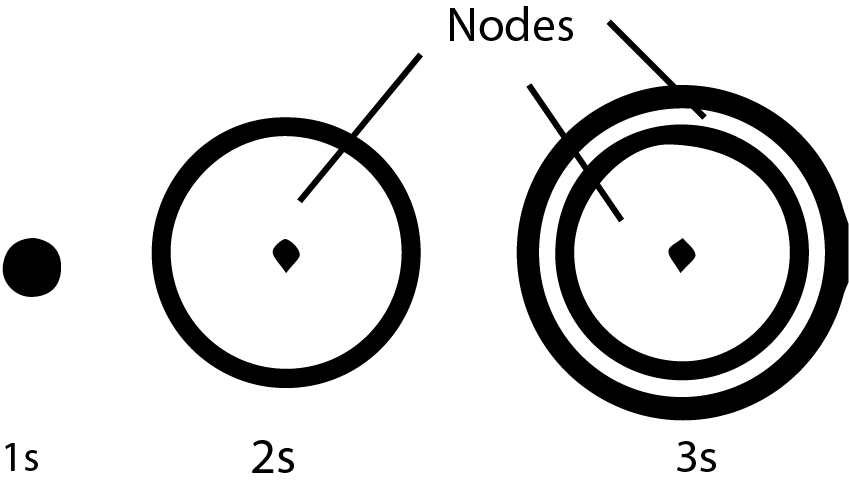
Q. Write a brief note on the Shapes of Orbitals.
There are three major orbitals s,p, and d are discussed.
1- Shapes of s-orbitals
s-orbital is spherical in shape and is usually represented by a circle.
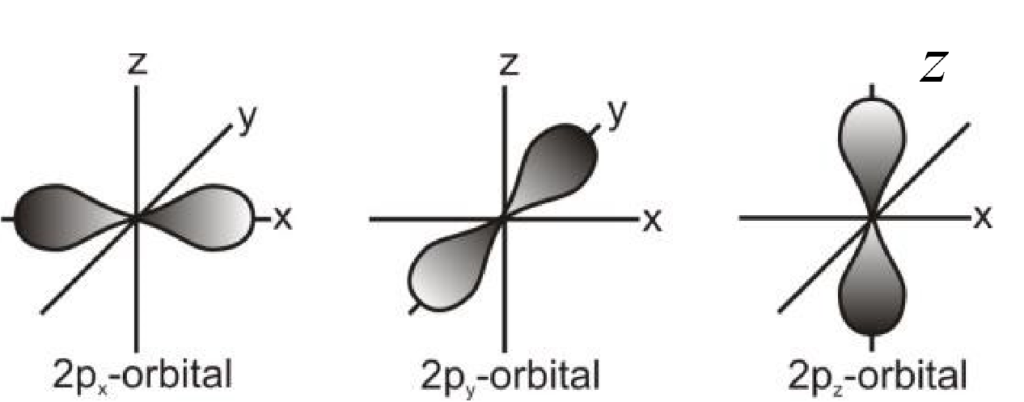
2- Shapes of p-orbitals
All three p-orbitals have dumb-bell shapes.
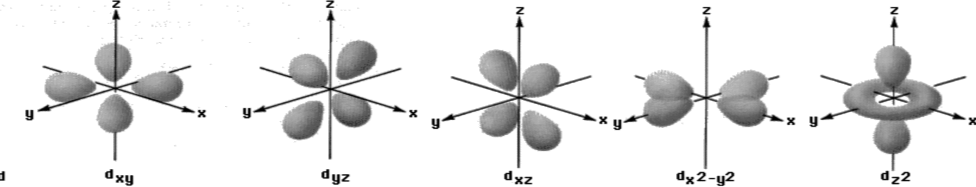
3- Shapes of d-orbitals
d-subshell have five values of magnetic quantum number. The lobes of first three d-orbitals lie
between the axis. The other lie on the axis.
Q. Name the rules which govern the electronic distribution in orbitals. Also explain them.
The arrangement of electrons in subshells or orbitals is according to the following rules.
- (n +l) rule
- Auf-bau principle
- Hund’s rule
- Pauli’s Exclusion Principle
1. (n +l) rule:
Gives the arrangement of electrons in subshells. It has two parts:
a) Electrons are filled in subshells in increasing order of their (n+l) values.
(b) If two subshells have the same (n+l) values, then the subshell with a low ‘n’ value will be filled first.
Ascending order of energy of sub-shells on the basis of (n + l) rule
1s < 2s < 2p < 3p < 3p < 4s < 3d < 4p < 5s < 4d < 5p < 6s < 4f < 5d < 6p < 7s
2- Auf-bau principle:
Electrons should be filled in subshells in order of their increasing energy values. It gives the same energy order as given by the (n +l ) rule.
3- Hund’s Rule:
When degenerate orbitals are available to more than one electron, then they are placed in separate orbitals with the same spin rather than in the same orbitals with opposite spin.
4- Pauli’s Exclusion Principle:
It is impossible for two electrons residing in the same orbital of a poly-electron atom to have the same
values of four quantum numbers.
OR
No two electrons in the same orbital can have the same spin (⇅).
Q. Differentiate between orbits and orbitals.
The volume of space where the chances of finding an electron is 95 % is called the atomic
orbital.
For example s, px, py, pz.
The path around the nucleus where the electron is revolving is called an orbit. The orbit is another term
which is used for the shell. An orbit can have one or more than one orbitals.
