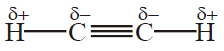Umair Khan Academy provides valuable notes for its students. Aliphatic Hydrocarbon is one of the most lengthy chapters in the book but you can summarize it in a few minutes with the help of the given short questions. Aliphatic Hydrocarbons comprise the chemistry of Alkane, Alkene and Alkynes.
INTRODUCTION OF ALIPHATIC HYDROCARBONS
Q1. Define Aliphatic hydrocarbons.
Open-chain or acyclic organic compounds that only contain carbon and hydrogen atoms are called aliphatic hydrocarbons.
Example: CH4 (Methane) CH3 – CH3 (Ethane)
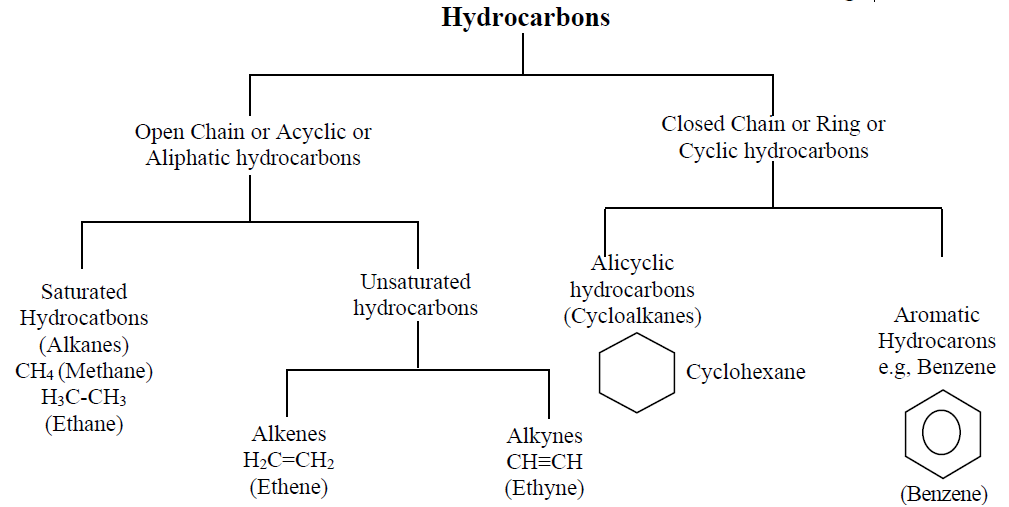
Q2. What is the Classification of Hydrocarbons?
Hydrocarbons are classified on the basis of
- Structure of chain
- Size of chain
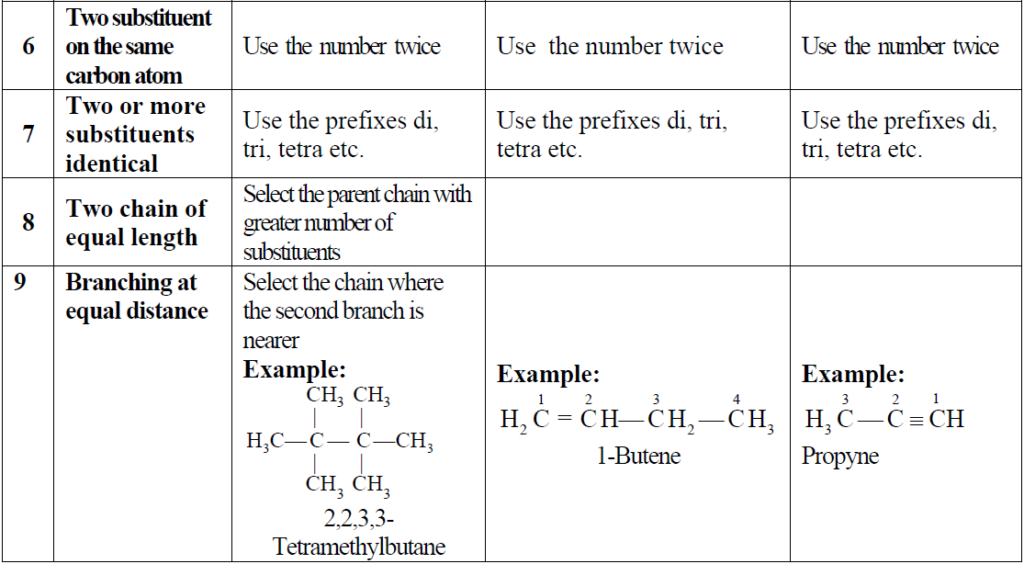
- Nature of the rings.
A brief description is given in the chart below.
NOMENCLATURE
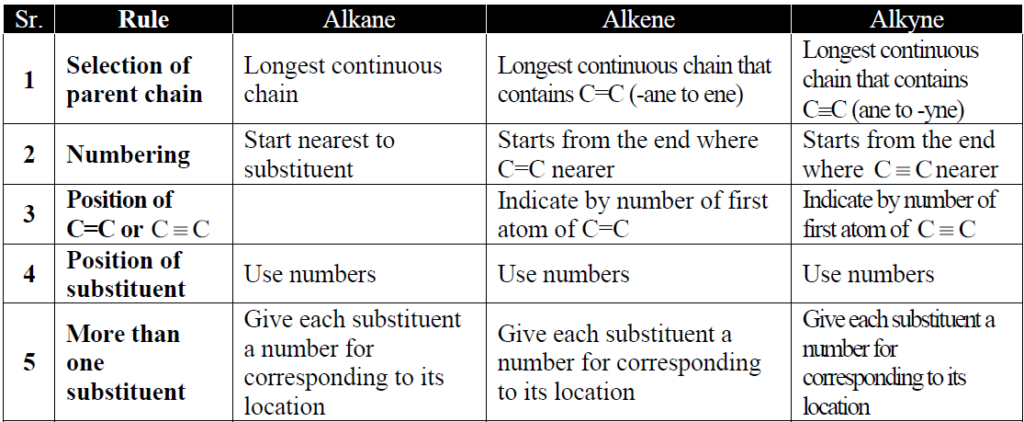
Q3. What is nomenclature and its basic types?
A system of giving a name to an organic compound is called Nomenclature and it is of two types.
- Common Or trivial name.
- IUPAC naming system.
Q4. What is Common or trivial naming?
Common or Trivial Names:
In the early days, the compounds were named on the basis of
- 1 Their History
- 2. The methods of preparation
- 3. Name of the person working on them
- 4. Sources
Example: Marsh gas found in marshy places.
The common or trivial names are applicable to all isomers
- Prefix n is used for unbranched or straight chains.
CH3―CH2―CH2―CH3 (n-Butane) - Prefix Iso is used for hydrocarbons having one methyl group as a branch at the second position of the chain.
- Prefix Neo is used for hydrocarbons having two methyl groups as branches at the second position of the chain.
Q5. Write a quick description of IUPAC Nomenclature.
Note:
If you are weak in naming Organic compounds then consider this explanation for full understanding of whole nomenclature from our book.
Nomenclature Of organic compunds.
Q6. Write the structural formula for two compounds: a) Vinyl acetylene and b) But-3-en-1-yne.
Both these compounds have the same structure:
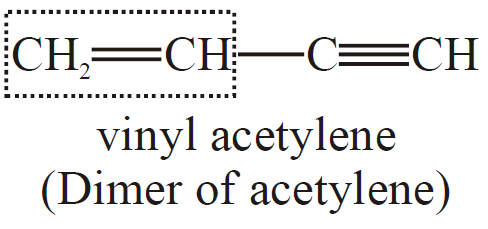
Q7. Write the IUPAC nomenclature of the following compounds.

ALKANES OR PARAFFINS
Q8. Defien Alkanes.
Saturated hydrocarbons with General Formula = CnH2n+2 are called alkanes. Alkanes are less reactive and, therefore, also called paraffins.
Q9. Write general methods of preparations for alkanes.
1- Hydrogenation of unsaturated hydrocarbons: (Sabatier-Sendern’s reaction)
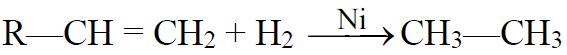
2- Alkyl halides
a) Reduction of Alkyl Halides:
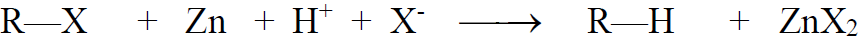
b) Hydrogenolysis:
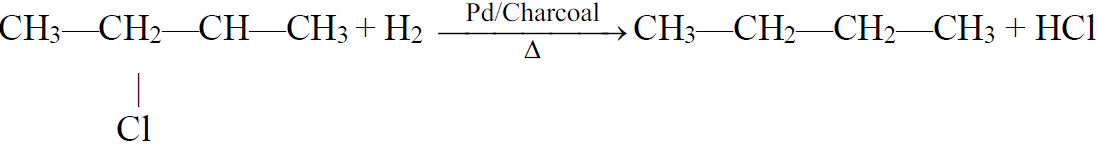
3- Decarboxylation of salts of monocarboxylic acids:
a) Decarboxylation by soda lime.
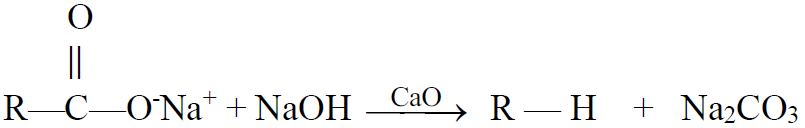
b) Kolbe’s electrolytic method:
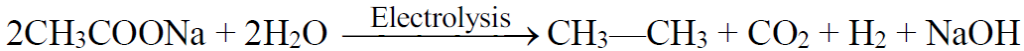
4- From Carbonyl compounds (Aldehydes and ketones):
a) Clemensen’s reduction:
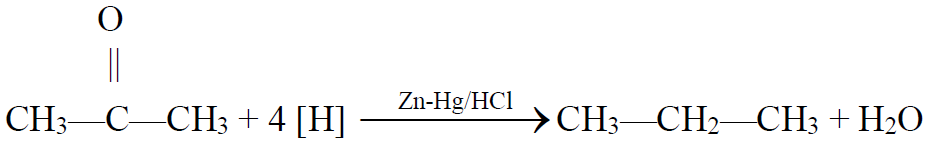
b) Wolf-Kishner’s reduction:
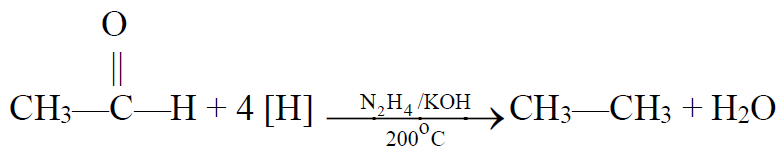
5- From Grignard Reagent:
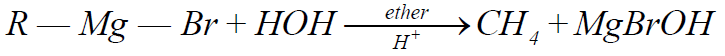
Q10. Write down the physical properties of Alkanes.
- Physical state:
C1 — C4 = colourless, odourless gases.
C5 — C17 =colourless, odourless liquids.
C18 and above = colourless, odourless waxy solids. - Solubility:
a) Insoluble in polar solvents but soluble in non-polar solvents
b) Solubility decreases with the increase in molar mass. - Physical constants:
Boiling points, melting points and density increase with the increase in the number of carbon atoms.
Q11. Why alkanes are less reactive?
Alkanes are less reactive due to the following reasons.
- They are non-polar due to the least electronegativity difference.
- All carbon-to-carbon and carbon-to-hydrogen are single bonds (σ-bonds).
Q12. Write down the Chemical reactions of alkanes.
The following are the most important reactions of alkanes.
1- Combustion:
a) Complete combustion:
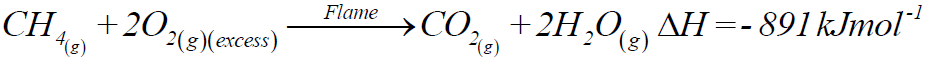
b) Incomplete combustion:
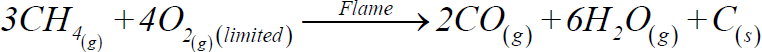
2- Catalytic oxidation:
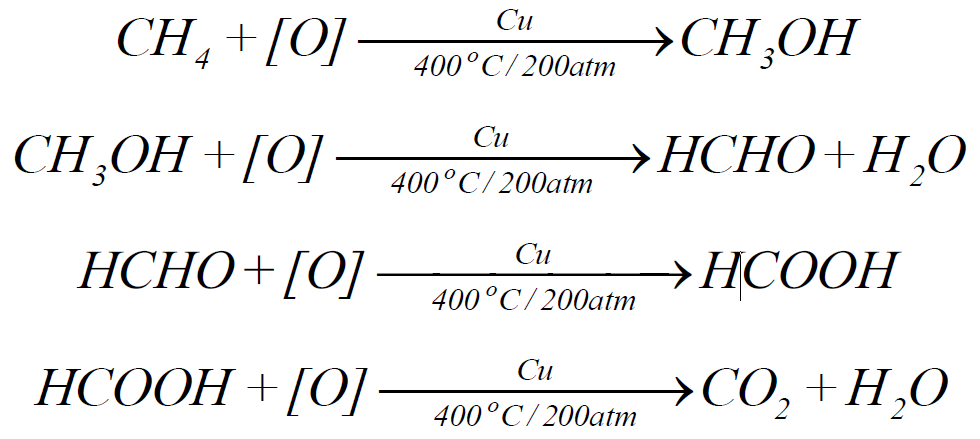
3- Nitration:
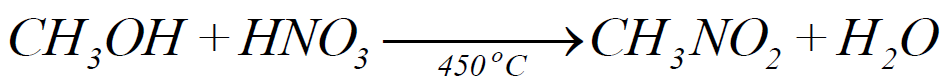
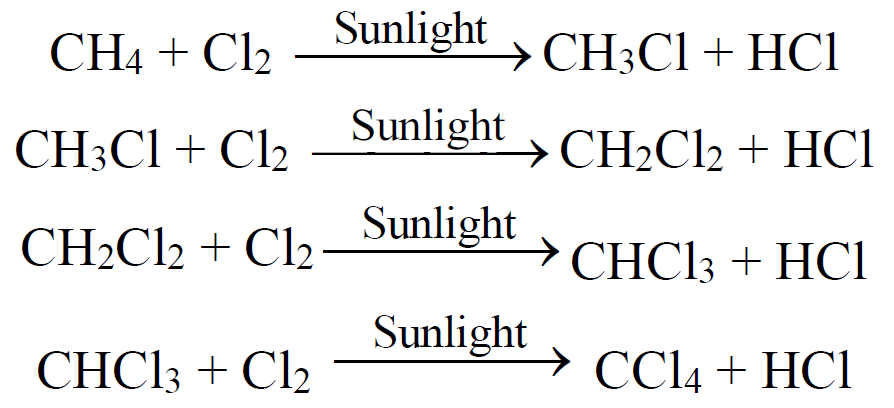
4- Halogenation: (Free Radical Reaction)
The reactivity order of halogens is as,
F2 > Cl2 > Br2 > I2
- Steps of free radical mechanism:
1. Initiation 2. Propagation 3. Termination
Q13. Write down the uses of methane.
- As a fuel and as an illuminating gas.
- For the preparation of methyl chloride, methylene chloride, chloroform and carbon
tetrachloride. - For the industrial preparation of methyl alcohol, formaldehyde and hydrogen cyanide.
- For the preparation of carbon black used in paints, printing inks and automobile tyres.
- To manufacture urea fertilizer.
Q14. What is Clemmensen’s reduction? Give its reaction.
The reduction of ketone to an alkane by using zinc amalgam and hydrochloric acid is
called Clemmensen’s reduction.
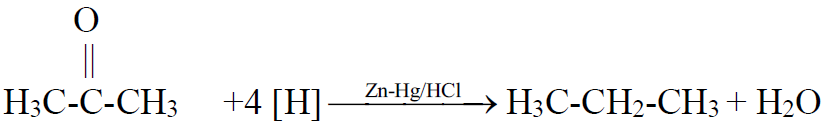
Q15. Why alkanes are the least reactive?
The least reactivity of alkanes may be explained on the basis of the non-polarity of bonds
forming them. The electronegativity difference between C and H is 0.4 which is not
appreciable and the bonding electrons between C-H and C-C are equally shared making them
almost non-polar. Furthermore, in alkanes, there are only sigma (σ) bonds. In the sigma (σ) bond the
electrons are very tightly held between the nuclei making the sigma (σ) bond very stable and
inert. Moreover, sigma (σ) electrons can neither attack any electrophile nor a nucleophile can
attack them.
ALKENES
Q16. Define alkenes
Unsaturated hydrocarbons with General Formula = CnH2n are called Alkenes or olefins.
Q17. Write general methods for the preparations of Alkenes.
1- Dehalogenation of Vicinal Dihalides:
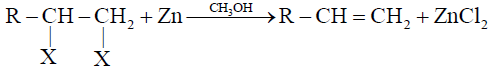
2- Dehydrohalogenation of Alkyl Halides:
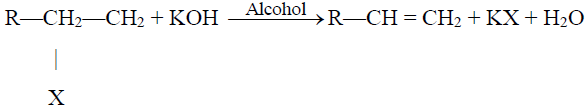
3- Dehydration of Alcohols:
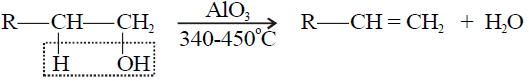
Q18. Write a few examples of dehydration agents.
P2O5 or P4O10, Conc. H2SO4 Conc. H3PO4 and Al2O3 are important examples of dehydrating agents.
Q19. Write an order of ease to dehydrate an alcohol, give a few examples.
Order of dehydration of alcohols:
Tertiary alcohol > Secondary Alcohol > Primary alcohol
1- Primary alcohol:
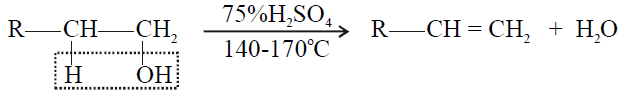
2- Secondary alcohol:
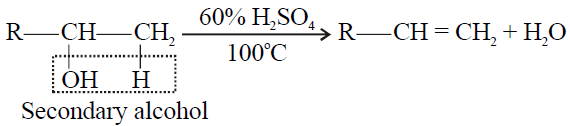
3- Tertiary alcohol:
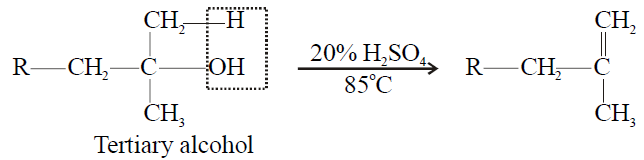
4- Electrolysis of salts of dicarboxylic acid (Kolbe’s Electrolytic Method):
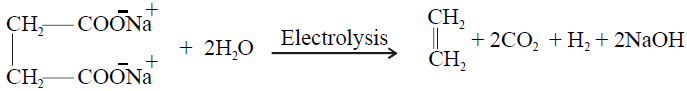
5- Partial Hydrogenation of Alkynes
Lindlar’s catalyst: Pd(BaSO4)/Quinoline
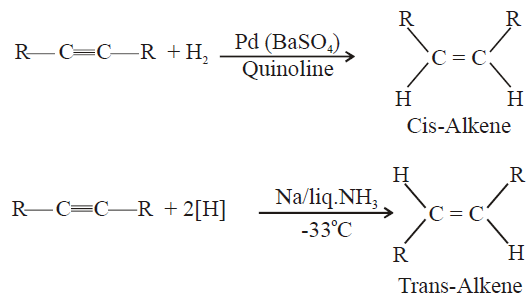
Q20. Write the physical properties of alkenes.
- Physical State:
C2 — C4 = Gases at room temperature.
C5 — C15 = liquids.
C16 and above = Solid. - Insoluble in water but soluble in alcohol.
- Characteristic smell and burn with a luminous flame.
- Weakly polar properties because of sp2 hybridization.
Q21. How is ethene converted into 1-butanol?
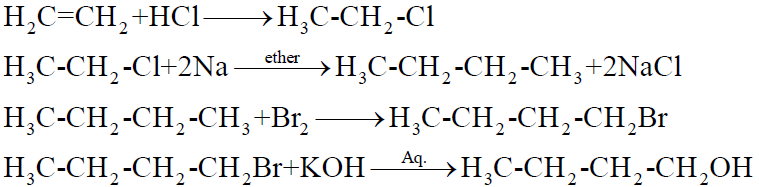
Q22. Write a few lines on the reactivity of the pi (π) bond.
Loosely held π-electrons are more exposed and an electrophile can easily attack on π –
electrons. π-bond is weaker and thermally less stable than σ-bond.
Q23. Write down the chemical reactions of alkenes.
A) Addition Reaction:
1- Hydrogenation:
Raney Nickel:
A form of Nickel prepared by treating Ni-Al alloy with caustic soda (NaOH).
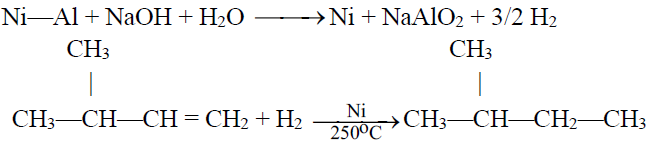
2- Addition of Hydrogen Halides:
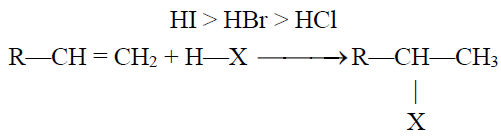
Q24. State the Markownikov’s Rule:
The negative part of the adding reagent adds to the carbon (constituting a double bond) which
has less number of hydrogen atoms. Only applicable when an unsymmetrical reagent is added to
an unsymmetrical alkene.
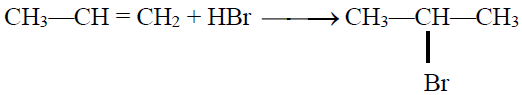
3- Addition of Halogens:
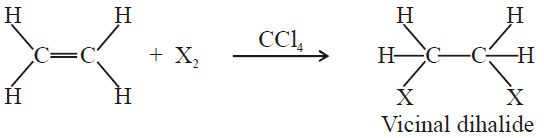
The reactivity order of halogen is;
F2 > Cl2 > Br2 > I2
4- Addition of Sulphuric Acid: (Conc. H2SO4)
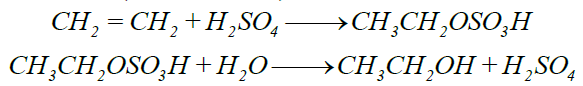
5- Addition of hypohalous acid: (HOX)
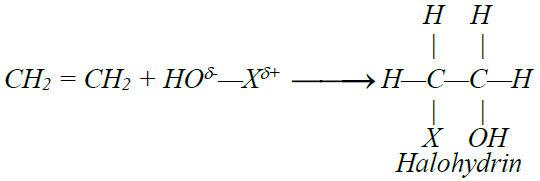
B) Oxidation Reactions:
1- Addition of oxygen:
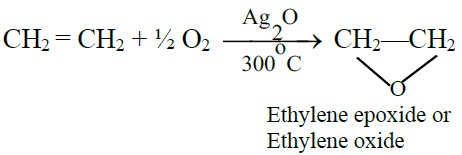
2- Hydroxylation: (Baeyer’s test)
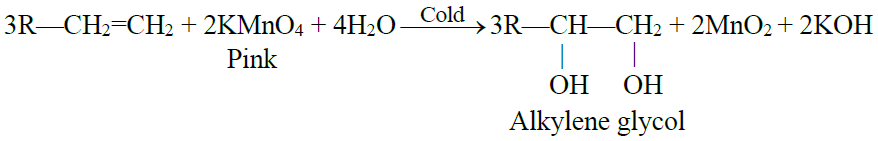
3- Combustion:
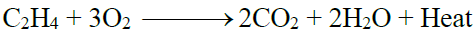
4- Ozonolysis:
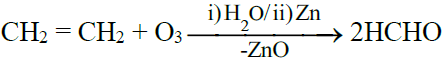
C) Polymerization:
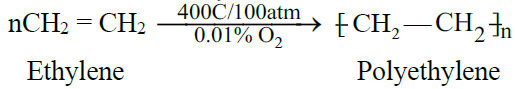
Q25. Write down the Uses of Ethene.
- For the manufacture of polyethylene plastic for making, toys, cables, bags, boxes etc.
- For artificial ripening of fruits.
- As general anaesthetic in hospitals.
- As a starting material for the preparation of a large number of chemicals for industrial use
such as glycols (antifreeze), ethyl halide, and ethyl alcohol. - To prepare mustard gas, a chemical weapon used in World War I
- Its name is due to its mustard-like odour.
- It is a vesicating agent. A substance that causes blisters on the skin is called a vesicating agent.
- It is a high-boiling liquid. It is dispersed as the mist of tiny droplets.
ALKYNES
Definition: Unsaturated hydrocarbons contain a triple bond called alkynes.
Q26. Write down the general methods for the preparation of alkynes.
1- Dehalogenation of Tetrahalides:
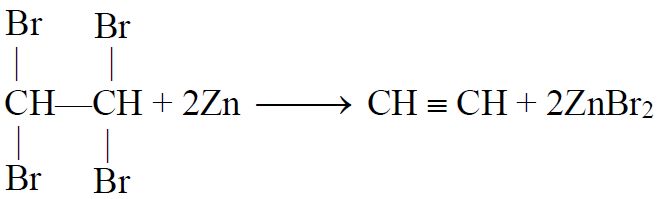
2- Dehydrohalogenation of vicinal dihalides:
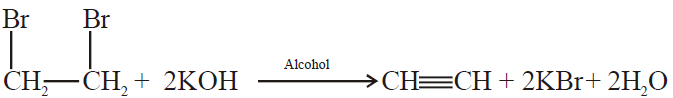
3- Electrolysis of salts of unsaturated dicarboxylic acids: (Kolbe’s electrolysis)
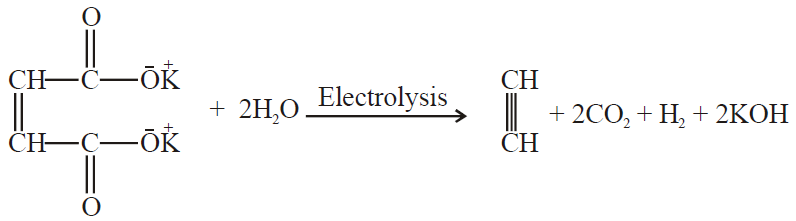
Q27. Write Industrial Preparation of Ethyne.
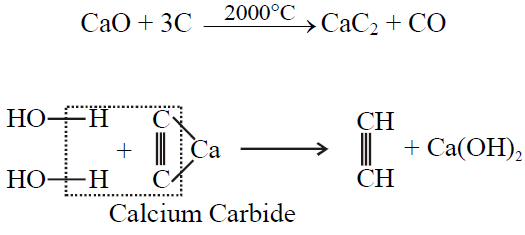
Q28. How mustard gas is prepared? Give its important use.
Mustard gas is prepared by treating S2Cl2 with ethene C2H4
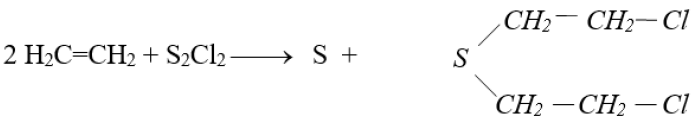
It is used in wars. It is a high-boiling liquid that is dispersed as a mist of tiny droplets. It is a powerful vesicant i.e. causes blisters.
Q29. What is Baeyer’s test? Give its use.
Hydroxylation: (Baeyer’s test)
The addition of a hydroxyl group to a double bond is called a Hydroxylation reaction. Alkenes react with 1% alkaline KMnO4 solution (mild oxidizing reagents) at low temperatures to form dihydroxy alkane. This dihydroxy alkane is called vicinal diol or glycol.
- The pink colour of the KMnO4 solution is discharged during the reaction.
- This reaction is used as a test for unsaturation in a molecule.
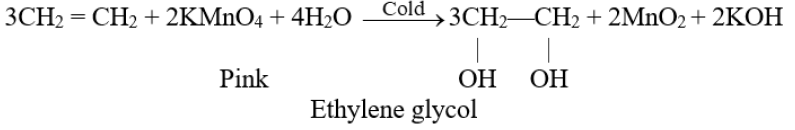
Q30. Write the physical properties of alkynes.
- Physical state
C2 — C4 = Colourless, odourless (except acetylene which has garlic like odour.) gases
C5 — C12 = Liquids
C13 and above = Solids. - The melting points, boiling points and densities increase gradually with the increase in
molecular masses. - They are non-polar and dissolve readily in solvents like ether, benzene and carbon
tetrachloride.
Q31. Write the reactivity of alkynes.
Alkynes are less reactive than alkenes due to the following reasons.
- A triple bond draws the nuclei close to each other and the molecule becomes stable.
- π-electrons are less exposed to electrophilic attack.
(A) Addition reactions:
1- Addition of Hydrogen:
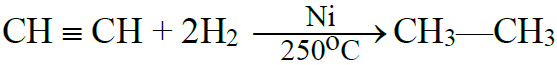
2- Addition of Halogens:
Reactivity Order:
F2 > Cl2 > Br2 > I2
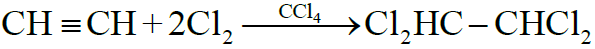
3- Addition of Halogen Acids:
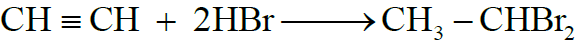
4- Addition of Water: (Hydration)
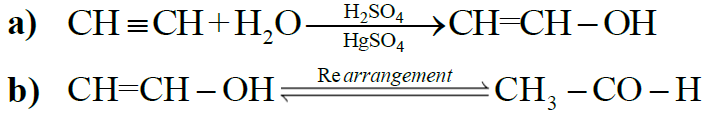
NOTE: All alkynes other than acetylene give ketones.
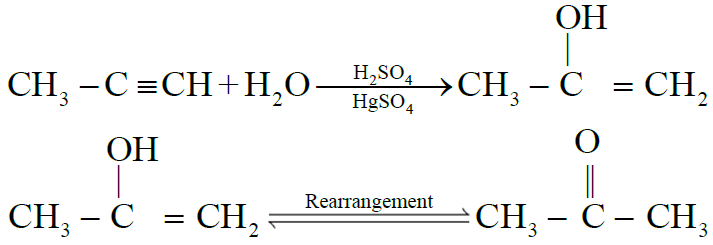
5- Addition of ammonia and hydrogen cyanide:
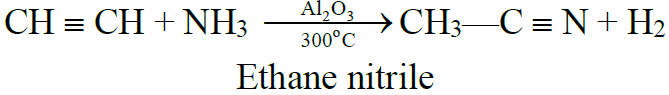
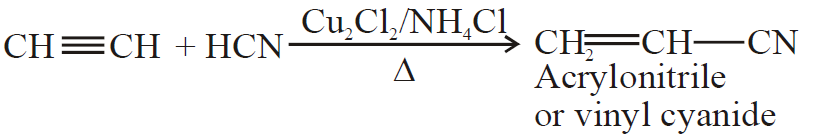
(B) Oxidation Reaction:
1- Oxidation of Ethyne
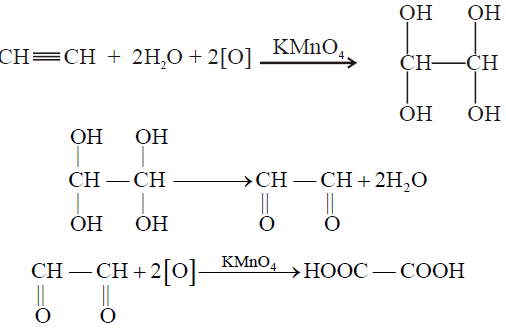
2- Combustion:
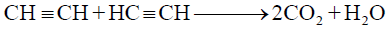
(C) Polymerization:
1- Formation of Divinyl Acetylene (Trimer) from vinyl acetylene:
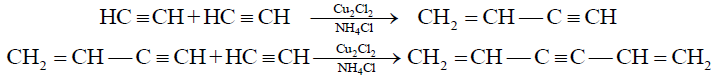
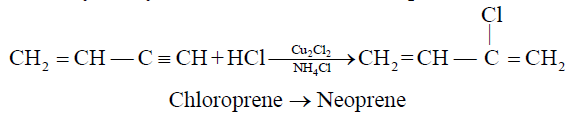
2- Addition of HCl to vinyl acetylene and formation of neoprene:
3- Formation of Benzene from acetylene:
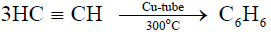
Q32. Define the Acidic Nature of Alkynes.
In ethyne and other terminal alkynes, a hydrogen atom attached to triply bonded Carbon atom is
acidic.
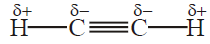
1- Reactions of alkynes with sodamide in liquid ammonia and sodium
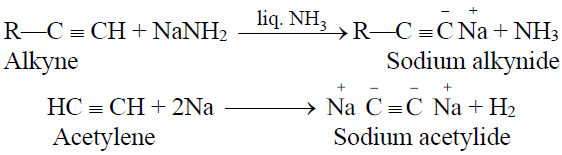
2- Identification of terminal alkynes:
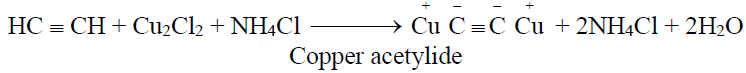
(Reddish brown ppt.)
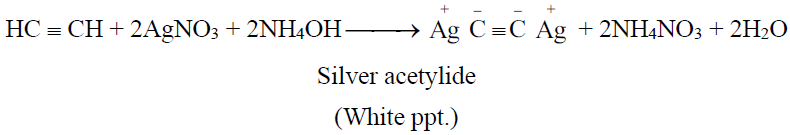
3- Regeneration of alkynes from the ppts. of alkynides:
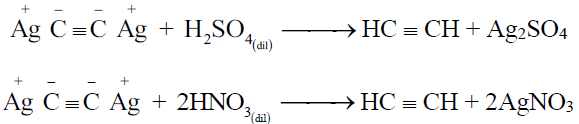
Q33. Write down the uses of ethyne.
- In oxyacetylene torch, which in turn is used for welding and cutting metals.
- For the preparation of alcohols, acetic acid and acetaldehyde.
- For the manufacture of polymers like PVC, polyvinyl acetate, polyvinyl ethers, orlon and
neoprene rubber. - For ripening of fruits.
Q34. Write down the Comparison of reactivities of Alkane, Alkene and Alkyne.
Reactivity order: Alkene > Alkyne > Alkane
- Alkene: Loosely held π-electrons are more exposed and an electrophile can easily attack
on π -electrons. π-bond is weaker and thermally less stable than σ-bond. - Alkyne: Triple bond draws the nuclei of bonded carbon atoms closer
Hence π-electrons are less exposed - Alkane: Paraffins = less reactive or less affinity
a) They are non-polar due to the least electronegativity difference.
b) All carbon-to-carbon and carbon-to-hydrogen are single bonds (σ-bonds). - Carbon to carbon bond lengths:
1. C-C = 154pm 2. C = C = 134pm 3. C ☰ C = 120 pm
Q35. How will you synthesize the following compounds from ethyne?
a) Benzene b) Chloroprene
a) Acetylene polymerizes to benzene on passing through a copper tube at 300°C.
Reaction
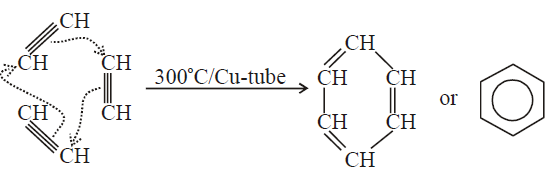
b) If HCl is added to vinyl acetylene, chloroprene is obtained which readily polymerizes to
Neoprene is used as synthetic rubber.
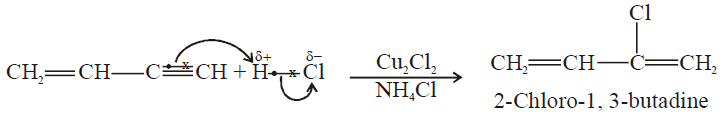
Q36. Justify ethyne shows an acidic character.
In ethyne and other terminal alkynes, a hydrogen atom attached to a triply-bonded Carbon
atom is acidic. The hydrogen atom is attached through sp-s overlap to a triply bonded Carbon atom.
sp orbital of triply bonded carbon atom has 50% s-characters in it that renders the sp carbon atom
more electronegative than Sp2 and Sp3. As a result, the sp-hybridized carbon atom of terminal
alkynes pulls the shared pair more strongly from attached hydrogen and makes it slightly acidic.