Class 12 Chapter 7 (Fundamental Principles of Organic Chemistry) Chemistry Notes are available. Important short questions from the examination point of view are given below. If you want detailed topics, you can watch the lectures here.
General Feature of Organic Compounds
Q 1: What is vital force theory? Who discarded this theory?
First of all, it was thought that organic compounds were synthesised in the bodies of plants and animals in the presence of vital force, which is present in plants and animals only. This concept was called the vital force theory. W.F Wholer in 1828, discarded this concept by preparing urea in the laboratory. He heated NH4CNO in the laboratory and got the urea.NH4CNO is an inorganic compound but urea is an organic compound.
NH4CNO ⇌ NH2CONH2
Q 2: Which organic compound was first of all prepared in the laboratory?
In 1828 F. Wohler prepared urea by heating ammonium cyanate in the laboratory. Urea is an organic compound present in animal urine.
NH4CNO ⇌ NH2CONH2
So Wholer’s work opened the way to preparing organic compounds in the laboratory. It was a revolution in the definition of organic compounds.
Q 3: What is a modern definition of organic chemistry and which of the elements other than carbon and hydrogen may be present in organic compounds?
According to the modern definition, the study of compounds containing carbon is called organic chemistry. The elements other than the carbon and hydrogen present in the various organic compounds are oxygen, sulphur, nitrogen, Phosphorus, and halogen in a few metals. Alcohol, glucose, sucrose, methane, and petroleum products all contain carbon in them.
Q 4: Mention those compounds of carbon which are not organic in nature.
The following are some compounds which have carbon in them but they are not thought to be organic in nature. These compounds are CO, CO2 CS2, HCN metal cyanide, metal carbide carbonates and bicarbonates of metals.
Q 5: Catenation is the property which makes carbon different from other elements of the periodic table. Justify it.
Carbon has the property to link with other atoms of carbon. This property of self-linkage is called catenation. Due to this property, a separate branch of chemistry called organic chemistry is developed. C2H6, C3H8, C10H22, C6H2O6, C12H22O11 and millions of other organic compounds in which carbon makes bonds with carbon to have long chains.
Q 6: How does the phenomenon of isomerism make the organic compounds greater in number?
The existence of more than one structure and having different physical and chemical properties with the same molecular formula is called isomerism. Butane has two isomers, pentane has three and hexane has 5. In this way, the number of possible molecules increases with the same number of carbon, hydrogen and those atoms, which are present.
Q 7: Organic compounds mostly give slow reactions as compared to inorganic compounds. Give reasons.
Organic compounds are mostly covalent in nature and insoluble in water. their polarity is not so pronounced, so the reactions are slow. ionic compounds of inorganic nature have ions in solution, that is why the reactions are fast.
Q 8: Organic compounds are mostly non-ionic in character. Give reasons.
In organic compounds, covalent bonds are there. The electronegativity values of carbon, hydrogen, Nitrogen, oxygen, and sulphur are 2.5, 2.1 3.0, 3.5 and 2.5 respectively. Whenever such atoms make bonds their electronegativity differences are much less than 1.7. Organic compounds are made up of such elements which have small differences of electronegativities. The bonds are very close to the covalent character and hence non-ionic.
Q 9: Which are the most important solvents and are used to dissolve organic compounds?
The important solvents are Ether, methanol, ethanol, benzene, carbon tetrachloride, chloroform, acetone and petroleum ether.
Q 10: How are organic compounds closely associated with the human body?
The substances like our food, clothing, medicines, vitamins, antibiotics, insecticides, hormones, pigments paper, ink, perfumes, soaps, detergent, petroleum and plastics etc are needed by the human being and they are all organic substances. It means that organic compounds are highly associated with the bodies of plants, animals and human beings.
Q 11: Organic compounds show similarity in behaviour. Comment.
Organic compounds are divided into various categories. it depends upon their functional groups. so there are homologous series according to the families of organic compounds. every member of the homologous series has similar behaviour.
Q 12: Complexity in structure is one of the important features of organic compounds. Comment.
Organic compounds are large in size. Their structures are complex. For example, the formula of starch is (C6H10O5)n. It means that thousands of C6H10O5 units are linked with each other to form one molecule of starch. Similarly, proteins are also complex molecules. Their molecular masses range from a few thousand to a million.
Sources of Organic Compounds
Q 13: Coal is one of the major sources of organic compounds. how do you justify it
Coal is found in the earth’s crust in the form of black deposits. These deposits are the remains of the vegetable matter. These vegetables (dead plants) were buried under the surface of the earth about 500 million years ago. Due to high pressure and temperature the vegetable matter changed into coal deposits. This happens due to the loss of the moisture and elimination of gases like Hydrogen and oxygen.
In addition to carbon hydrogen and oxygen amounts of nitrogen and sulphur are. Some amounts of moisture and mineral impurities are also there. Due to the bacterial and chemical action on wood, it was converted into peat.
Q 14: How is peat converted into other forms of coal?
Peet is converted to other forms of coal as shown below.
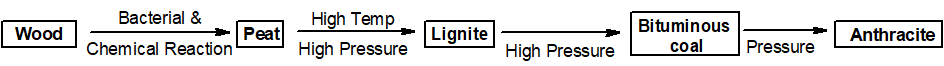
Q 15: What is carbonization?
It involves heating coal at about 1270 K in the absence of air. It is also called destructive distillation of coal or paralysis.
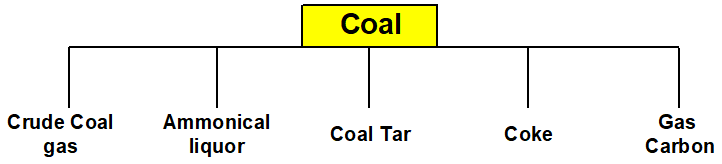
Q 16: Indicate five fractions which are obtained from the destructive distillation of coal.
When coal undergoes destructive distillation between 1000 to 1400℃ the following five fractions are obtained.
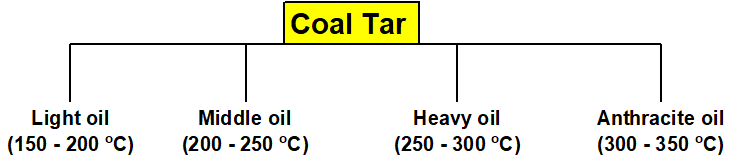
Q 17: What is Coal Tar? Give its fractions.
Coal tar is one of the fractions obtained from the destructive distillation of coal. When it undergoes fractional distillation four fractions are obtained having approximately 250 organic compounds.
Q 18: What is the composition of natural gas?
Natural gas consists of 60 – 95% Methane. It has small amounts of ethane, propane and butane. It also contains CO2, H2S and Nitrogen gas.
Q 19: Give important uses of natural gas.
It is extensively used in kitchens at home and in many Industries as a source of energy. Natural gas is used as a fuel to produce carbon black and acts as raw material for the manufacture of urea. It is also used for power generation in the cement and Fertilizer industries.
Q 20: What is petroleum? Give its origin.
petroleum is a naturally occurring brown or greenish-black viscous oily liquid which is obtained from the earth’s crust. According to the modern theory, it is believed that petroleum has a biological origin. Petroleum is formed due to the decay and decomposition of marine animals. The vegetable organisms of the prehistoric forest have also been decayed and decomposed. It is thought that the prehistoric forest and sea animals got buried underground. This biological matter found very high pressure and temperature in the interior of the earth for years. This process is responsible for decomposition under the influence of radioactive substances or by bacterial agencies.
Q 21: Name the fractions which are obtained by fractional distillation of Petroleum.
When petroleum undergoes fractional distillation various fractions such as natural gas, petroleum, naphtha, gasoline, kerosene oil, diesel oil, lubrication oil and asphalt etc are obtained. These fractions have different ranges of boiling points. These fractions are used as fuels and starting materials for Petroleum products.
Q 22: What is an oil refinery? Mention oil refineries in Pakistan.
Those industries which purify the petroleum and separate different components are called oil refineries. At present four oil refineries are in operation in our country.
- Attock oil refinery which is situated in Rawalpindi has about 1.25 million tons of capacity to refine the oil annually.
- Two oil refineries are located in Karachi. They have 2.13 million tonnes of capacity for oil refining.
- The Pak-Arab refinery is located at Mehmud Kot near Multan.
Q 23: Give the boiling point range and composition of kerosene and naphtha.
Kerosene oil has a boiling point range of 175 – 325℃ and its hydrocarbons vary from C8H18 – C14H30.
Naphtha obtained from distillation of the petroleum has a number of confusing names which are unscientific and chemically erroneous. These names are petroleum ether, petroleum spirits, mineral oil or mineral spirit. their melting point may be between 50 to 60℃ and has compounds above C23H48.
Cracking
Q 24: What is cracking of Petroleum? Why do we need cracking of Petroleum?
The process of decomposition of less volatile higher hydrocarbons into more volatile lower hydrocarbons with the application of heat and catalyst is called cracking. Fractional distillation of petroleum only gives 20% gasoline. The rest of the deficiency is compensated by cracking. Approximately 50% of gasoline is now prepared by this method.
Q 25: Give the products of the cracking of n-butane.
n-butane gives different types of products on cracking.
- 1-Butene
- 2-Butene
- Ethane
- Ethene
- Propene
- Methane
Q 26: What is thermal cracking?
That cracking which is carried out by the application of heat and pressure is called thermal tracking. In the liquid phase, the temperature of 475 to 530℃ is maintained with a pressure of 7 – 70 atm. In vapour phase cracking, the temperature is 600℃ and a pressure of 3.5 – 10.5 atmospheres is required.
Q 27: What is catalytic cracking?
catalytic cracking is done in the presence of a catalyst. The temperature and pressure are comparatively low. A typical catalyst used for this purpose is a mixture of silica (SiO2) and Alumina(Al2O3). The mixture of these two substances gives us aluminium silicate. catalytic cracking produces gasoline of higher octane number. In this way the gasoline of better quality is obtained.
Q 28: What is steam cracking?
Higher hydrocarbons are converted to vapour and mixed with steam. This mixture is heated for a short time to about 900℃ and then cooled rapidly. This cracking gives us lower unsaturated hydrocarbons.
Knocking
Q 29: What do you mean by knocking of Petroleum?
Knocking is a metallic sound produced during the combustion of gasoline in internal combustion engines. The gasoline having a lower Octane number produces knocking. It lowers the efficiency of the engine.
Q 30: How do you define Octane number and how it can be improved?
Octane number reflects the quality of gasoline and has more number of branched chain hydrocarbons such as iso-octane.
The Octane number of gasoline is improved by the process called reforming. It involves the conversion of straight-chain hydrocarbons into branched chains by heating in the absence of oxygen and in the presence of a catalyst.
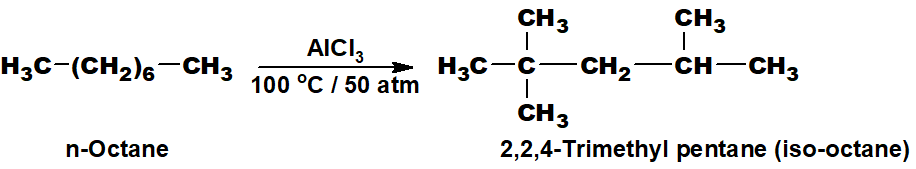
Q 31: What is the reforming of Petroleum? Give one example.
Reforming is the process of conversion of straight-chain alkanes into branched-chain alkanes. n-Octane can be converted into iso-octane when it is heated at 100 ℃ and at 50 atmospheric pressure in the presence of aluminium chloride as a catalyst.

Types of Organic Compounds
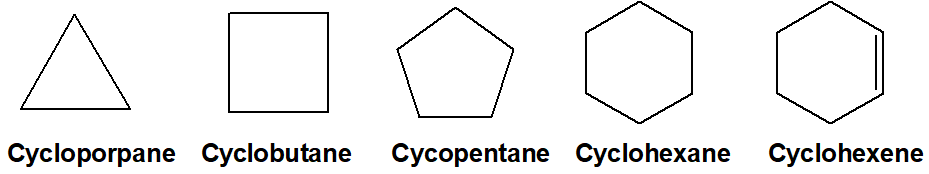
Q 32: Give at least 5 compounds which are homocyclic and are carbocyclic but are not aromatic.
When a cyclic compound has the same atoms to form a cyclic ring then they are called homocyclic. Aromatic compounds are also cyclic. The following are carbocyclic but not aromatic,
Q 33: Five four examples of aromatic heterocyclic compounds and two examples of homocyclic aromatic compounds.
The best and simple examples of aromatic heterocyclic compounds are as follows. These are five-membered and one is six-membered. There are millions of derivatives of these compounds.
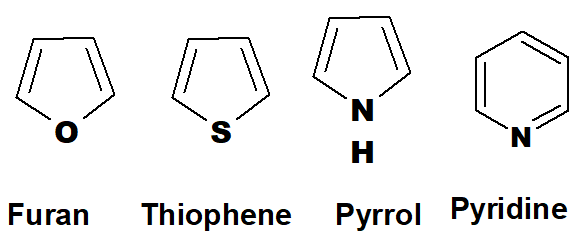
The aromatic homocyclic compounds are derivatives of benzene.
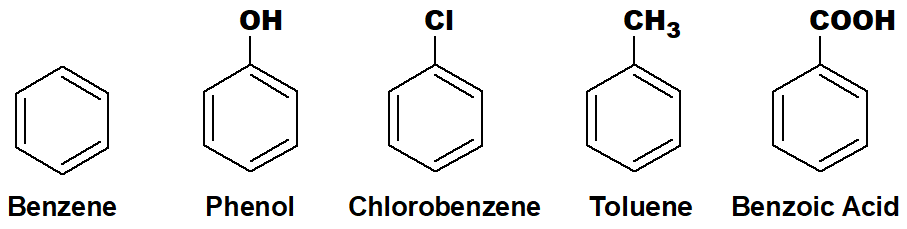
Q 34: What are aromatic hydrocarbons? Give two examples.
Those organic compounds which contain at least one benzene ring with an alkyl or aryl group are called aromatic hydrocarbons.
Aromatic compounds have alternate single and double bonds. In the case of the benzene ring, any one of the hydrogen or all the hydrogens can be substituted by an alkyl or aryl group to make the aromatic hydrocarbons.
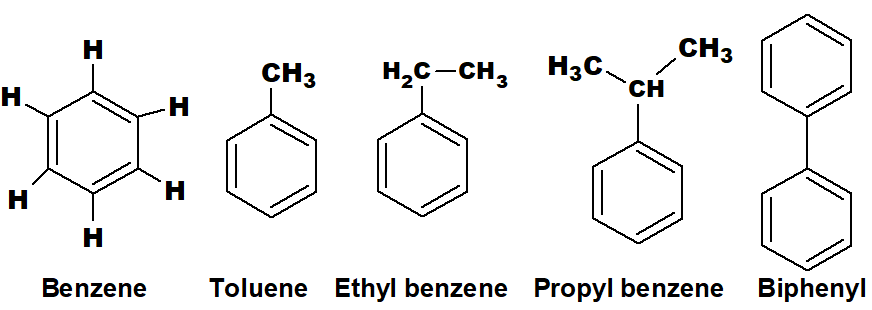
There are thousands of aromatic hydrocarbons. However, certain compounds are aromatic, but their aromaticity is due to a ring or rings other than benzene.
Q 35: which are two important branches of acyclic compounds?
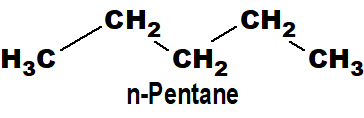
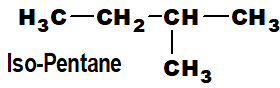
Those organic compounds which have an open chain of carbon atoms are called acyclic compounds. Acyclic compounds have a linear chain and a branched chain.
Q 36: Classify open-chain compounds with examples.
There are two types of such compounds.
- Straight chain
- Branched-chain
Q 37: What is meant by functional group? Name and represent four functional groups containing oxygen.
An atom or group of atoms in a molecule which give characteristic properties to compounds is called a functional group.
| Group Name | Functional group |
| Alcoholic | -OH |
| Carbonyl | -CO- |
| Carboxylic | -COOH |
| Aldehydic | -CHO |
Q 38: What are homologous series? Give their Characteristics.
The numbers of homologous series contain the same elements and the same function group. They have the same general formula and members differ by -CH2– units. Their properties and preparations are identical and their physical properties change gradually. Alkanes, alkene, alkyne, alcohols, carboxylic acid etc all give homologous series.
Structures of Organic Compounds
Q 39: What is sp3 hybridization? Justify with reference to CH4.
Carbon is the central atom of CH4. The electronic distribution of carbon is as follows.
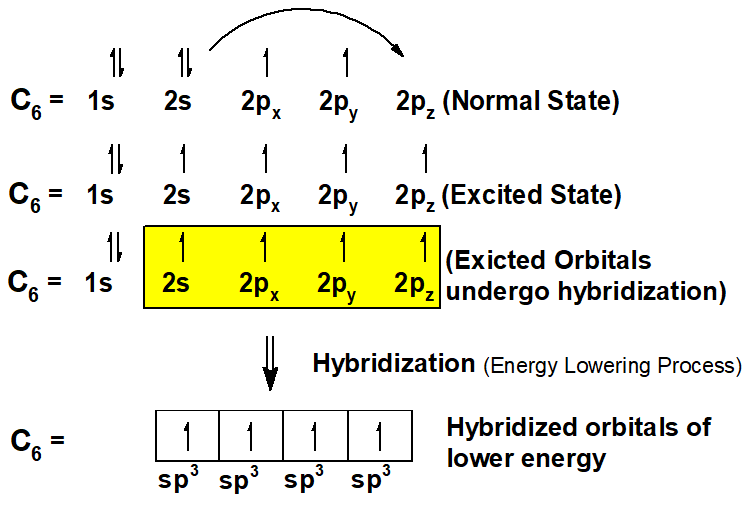
sp3 hybridised orbital has 25% s-character and 75% p-character. Each sp3 orbital has two lobes. One of the lobes is very very small so it is ignored while drawing the structure of the sp3 orbital. The mixing up of the orbitals to form new orbitals of equal energy is the energy of the lowering process. This energy lowering more than compensates that energy which is absorbed during the promotion of the electron from 2s to 2pz orbital.
Q 39: Draw the structure of CH4 and CCl4 indicating the sp3 hybridization of the central carbon atom.
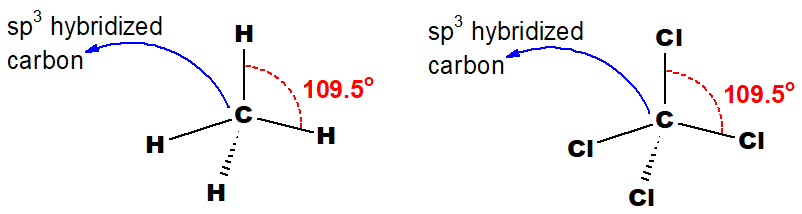
Both structures are perfectly tetrahedral and central carbon atoms are sp3 hybridized.
Hybridization can be represented as follows.

Q 40: Pi (π) Bond is weaker than Sigma(ઠ) bond. justify it.
π bond is formed by parallel overlapping of p orbitals. Two electrons give two lobs above and below the sigma bond. π bond is a weaker bond and less energy is required to break it. Its electronic cloud is more diffused as compared to electrons of the sigma bond.
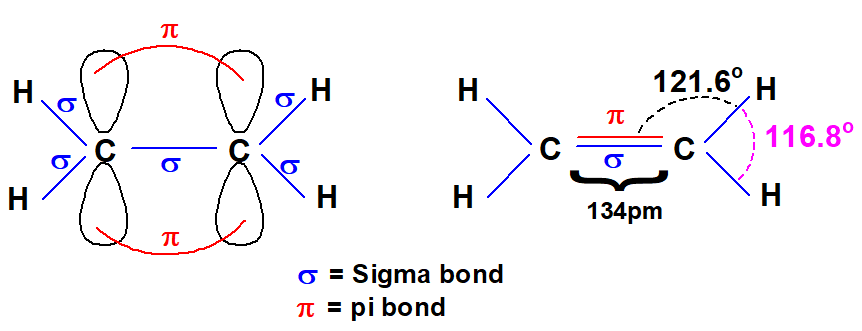
The bond angles should have been 120° but they differ due to the different sizes of electronic clouds of bonds. The cloud of 4 bonding electrons of C=C occupies more space and pushes electron pairs of carbon-hydrogen bond closer to each other.
Q 41: Draw the structure of C2H2 and indicate the bond length and bond angles.
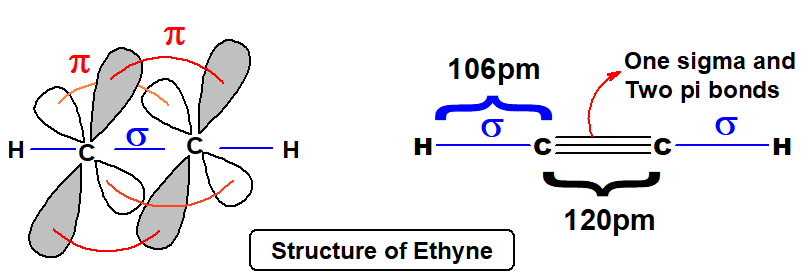
Ethyne is a linear molecule.
Structural Isomerism
Q 42: What is isomerism? Give the names of the four structural isomerism.
That phenomena in which two or more compounds have the same molecular formulae but differ at least in some of their physical or chemical properties is called isomerism. There are five types of structure isomerism.
- Chain isomerism or skeletal isomerism
- Positional isomerism
- Functional group isomerism
- Metamerism
- Tautomerism
Q 43: Give examples of positional isomers in alkenes, alkynes and xylene.
When the position of C = C is different then they are positional isomers.
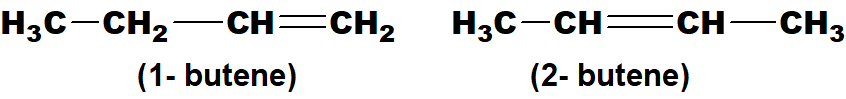
Above mentioned examples are isomers of butene as the position of double bonds is different.
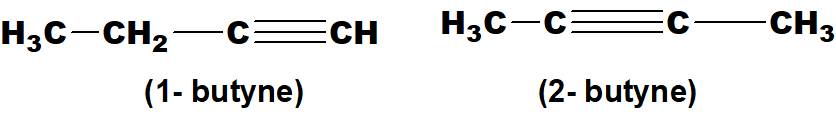
Above mentioned examples are isomers of butyne as the position of triple bonds is different.
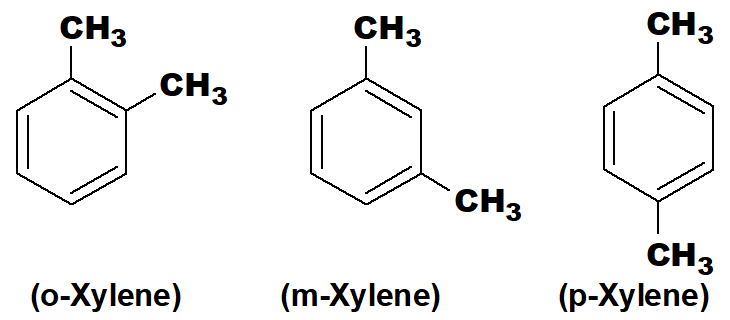
Xylene has three positional isomers.
Q 44: Give two examples of functional group isomers.
Those isomers which differ in the nature of the functional group are called functional group isomers.
For example.
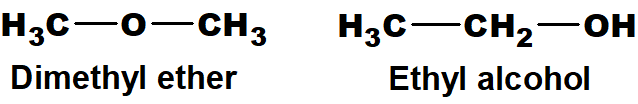
Above mentioned ether and alcohol are functional group isomers.
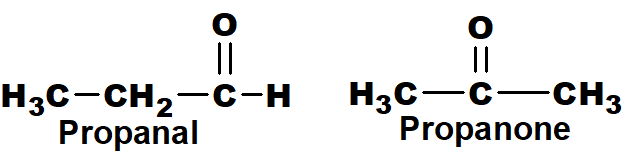
Above mentioned aldehyde and ketone are functional group isomers.
Q 45: What is metamerism? Give one example.
Those structural isomers in which a polyvalent atom is attached with different types of alkyl groups. Diethyl ether (CH3CH2OCH2CH3) and methyl n-propyl ether (CH3OCH2CH2CH3) are metamers of each other. Similarly, CH3CH2COCH2CH3 and CH3COCH2CH2CH3 are metamers of pentanone. These ketones have the molecular formula C5H10O.
Geometrical Isomerism
Q 46: Why is restricted rotation necessary to show geometrical Isomerism?
Single bonds are always rotating, so molecules can attain any conformation. If there is a double bond then the presence of the pi bond does not allow the sigma bond to rotate and rotation is hindered. In this way, cis and trans geometrical isomers can exist.
Q 47: How does the restricted rotation of cyclic compounds give birth to geometrical isomerism?
The bonds which are making the cyclic rings are not rotatable due to cyclic structure, so cis and trans geometrical isomers are possible.
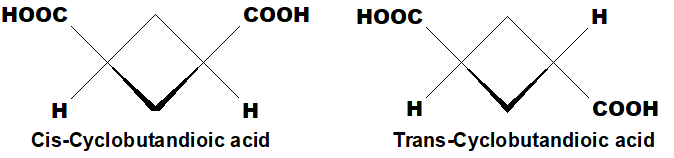
In cis compound both -COOH groups are on the same side of the cyclobutane ring. In trans, they are on opposite sides.
Q 48: 1-butene does not show geometrical isomerism, but 2-butene does. Give reasons.
In 1-butene two similar hydrogen atoms are present at the double-bonded carbon atom number 1 so it cannot show geometrical isomerism.
Both (i) and (ii) are similar compounds. They have different physical and chemical properties.
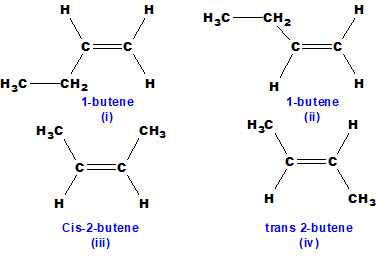
Q 49: 2-butyne does not show geometrical isomerism, but 2-butene does. Give reasons.
In 2-butyne only one group is attached with sp-hybridised carbon atom. So, it can not show geometrical isomerism.
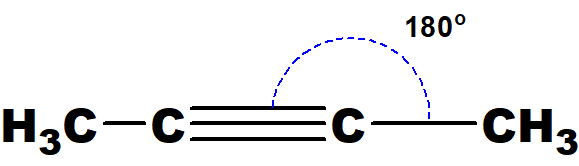
In 2-butene there are two groups at each sp2– carbon atom and can have two possible ways in space to get attached with sp2-carbon atoms.
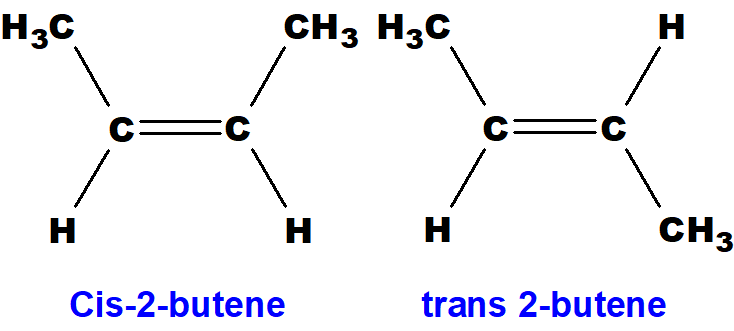
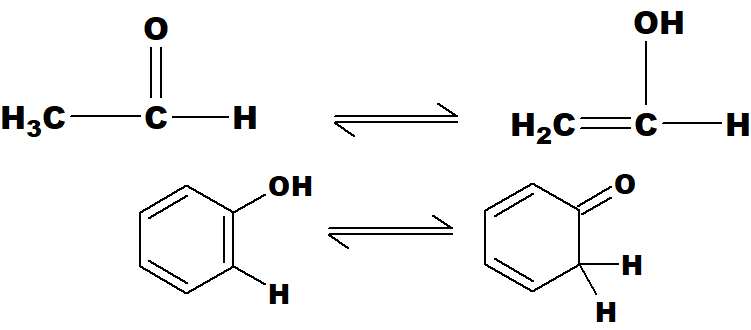
Q 50: Define tautomerism with an example.
It is a type of isomerism in which an atom is transferred from one part of the molecule to the other within the same molecule.
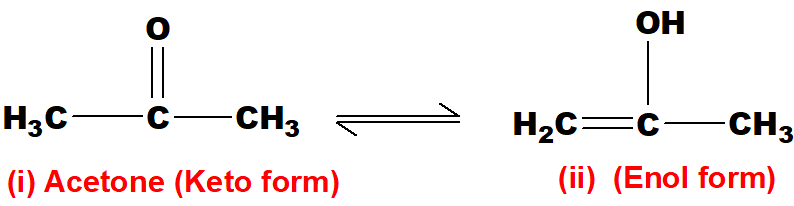
The H-atom has transferred from carbon to oxygen atom. this is called keto-enol tautomerism. (i) and (ii) are tautomers of each other.
Q 51: Give two tautomeric structures for each of CH3CHO and phenol.