This post provides you with full revision and preparation for experimental techniques, a chapter from class 11 chemistry F.Sc, in terms of quizzes, short questions and Video Lectures.
Attempt quizzes from
1. [SET 1]
2. [SET 2]
3. Learn full chapter [short questions]
4. [Theory]
Start your quiz by clicking the Next button below [SET 1]
Start your quiz by clicking the Next button below [SET 2]
Learn full chapter by revising short questions.
Q No. 1: Define Experimental Techniques in chemistry.
Experimental techniques are performances that are carried out to do analysis for under observation substances with the help of instruments in the chemical lab.
Q No. 2: Define analytical chemistry?
A branch of chemistry that deals with the study of qualitative and quantitative analysis is called analytical chemistry.
Q No. 3: Define qualitative analysis.
An analysis which is used to detect the presence or absence of any required element in a given substance is called qualitative analysis. e.g. potassium is present in a given soil sample.
Q No. 4: Define quantitative analysis.
An analysis that provides the information about required elements in a given substance in amounts (numbers) using experimental techniques. e.g. 3g potassium in 1m2 of soil sample.
Q No. 5: Write down the steps involved in quantitative analysis.
There are four major steps involving quantitative analysis.
- Collection of sample
- Separation of specific component from sample
- Relevant experimentation, calculations
- Find out the results.
Q No. 6: Define filtration.
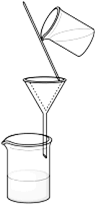
A process by which insoluble solid particles are removed from a solution through filter media (filter paper or crucibles) is called filtration.
Q No. 7: Define residue.
Material left behind on filter paper after the process of filtration is called the residue.
Q No. 8: Define filtrate.
A liquid that is obtained in a beaker after the process of filtration is called the filtrate.
Q No. 9: How can we run the process of filtration smoothly?
Following measurements should be taken.
- Stem of the funnel should be full of liquid during whole process.
- Stem of the funnel should be long enough to reach in the beaker, collecting filtrate.
- Stem of the funnel sides should touch the walls of beaker, therefore, no splash occurs.
Q No. 10: Write down the process of folding filter paper.
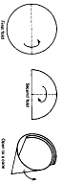
Following is the procedure to fold a filter paper correctly.
- Take a round filter paper
- Fold it from middle along the diameter.
- Again fold it in such a way that edges should not match equally, slightly unequal.
- Now start opening from conical side having four folds, three folds should be separated from one fold to make it open cone like structure.
- Apex angle should be according to funnel so that it can be adjust in funnel correctly.
Q No. 11: What is fluted filter paper?

A simple round filter paper is folded in too many folds (i.e. 16 folds) in such a way that edges make alternate elevations and depressions as shown in the figure.
Filtration through fluted filter paper is more rapid as compared to simple conical filter paper.
Q No. 12: Write a few lines on Gooch Crucible.

The Gooch crucible is made up of porcelain with a perforated bottom. Its bottom should be prepared by retaining paper pulp, cotton wool, asbestos mat or a filter paper of its size according to the type of material to be filtered. In the case of acids and KMnO4, filter paper can’t be used.
The process of filtration can be increased by placing the Gooch crucible on the suction filtering apparatus.
Filtration through the Gooch crucible is useful for separating precipitates; need to be ignited at high temperature.
Q. No. 13: Why Gooch crucible is preferred over filter paper?
Concentrated acids like HCl and oxidizing agents like KMnO4 solutions react with filter paper and no filtration takes place. To avoid this problem asbestos mat is used in place of filter paper.
Q. No. 14: what is Sintered glass crucible?
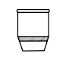
A crucible that is used to filter every kind of material without any preparation as needed in the Gooch crucible is called Sintered glass crucible. It has a porous glass disc sealed into the bottom and is very easy to use.
Q. No. 15: A water-insoluble organic compound aspirin is prepared by the reaction of salicylic acid with a mixture of acetic acid and acetic anhydride. How will you separate the product from the reaction mixture?
Aspirin is separated from the reaction mixture by filtration. The reaction mixture is added to cold water. The aspirin forms crystals and other products remain in solution. Finally, aspirin is filtered from the water by sintered glass crucible.
Q. No. 16: Define crystallization.
An experimental technique that is used to separate a pure substance in the form of crystals from the impure mixture is called crystallization.
Q. No. 17: Define crystal.
A specific geometrical shape of a substance is called a crystal.
Q. No. 18: What is a principle of crystallization?
The basic principle of crystallization is the fact that the solute should be soluble in a suitable solvent at high temperature and the excess amount of the solute is thrown out as crystals when it is cooled.
Q. No. 19: Write down the major steps involved in crystallization.
The major steps are as follows.
- Choice of solvent.
- Preparation of saturated solution.
- Filtration of impurities.
- Cooling of hot filtrate.
- Collection of crystals.
- Drying of crystals.
- Decolourization of crystals.
Q. No. 20: Give the main characteristics of the solvent used for crystallization.
Characteristics of good solvent are as follows.
- Ideal solvent should dissolve a large amount of the substances at its boiling point and only a small amount at the room temperature.
- There should be no chemical reaction between solute and solvent.
- Either it should not dissolve impurities or impurities should not crystallize out with required solute.
- On cooling it should give out a large amount of crystals of pure substance.
- It should be cheap.
- Good solvent should be separate easily and easy to use.
Q. No. 21: Name the mostly used solvents in crystallization.
Mostly the following solvents are used.
1. Water 2. Rectified spirit. 3. Absolute alcohol
4. Ether 5. Acetone 6. CCl4
7. Chloroform 8. Acetic Acid 9. Petroleum Ether
Q. No. 22: Define mother liquor?
A solution remaining after the formation of crystals is called mother liquor.
Q. No. 23: How do vacuum desiccators work to dry the crystals?

Vacuum desiccators are used to dry the crystals in a safe way. Fresh and wet crystals are spread over the watch glass and kept in a vacuum desiccator for several hours. Drying agents are also used in the bottom of desiccators. Some are listed as, Anhydrous CaCl2, silica gel and P2O5.
Q. No. 24: How the undesirable colour is removed from freshly prepared crystalline substances.
Boiling of coloured substances with the appropriate amount of animal charcoal in the solvent can decolourize undesirable colours. In this way, coloured impurities are absorbed by the charcoal and the pure substance is crystallizing out on cooling.
Q. No. 25: Define Crude products? And why there is a need to crystallize it.
A compound with impurities is called a crude product. And to eliminate these impurities to obtain the pure substance, crystallization is carried out.
Q. No. 26: Define sublimation.
Sublimation is a process by which a solid upon heating directly changes into vapours without passing through the liquid phase and these vapours can be condensed to form solid again. This solid is called sublimate. Common examples of sublimate are ammonium chloride, iodine, naphthalene, benzoic acid etc.
Q. No. 27: Define Sublimand and sublimate.
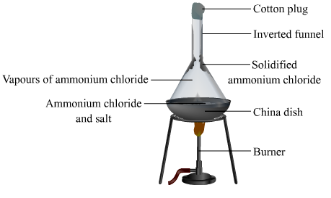
Sublimand: The solid substance being sublimed is called Sublimand.
Sublimate: The pure solid obtained after sublimation is called sublimate.
Q. No. 28: What is the main function and limitation of sublimation?
By this process of sublimation certain substances can be purified. It is only suitable for those substances which have high V.P than their melting point.
Q. No. 29: Write down the process of sublimation for the purification of impure naphthalene.
Following steps should be taken to purify naphthalene by the process of sublimation.
- Impure naphthalene is placed in watch glass covered with the inverted funnel.
- Open end of funnel stem is covered with cotton plug.
- Naphthalene is heat slowly over a sand bath.
- Funnel is cooled with wet cotton.
- The pure naphthalene deposits on the inner side of the funnel leaving behind impurities in the watch glass.
- In this way naphthalene is purified.
Q. No. 30: What is solvent extraction? When it is applicable?
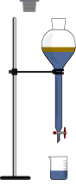
It is a technique, in which a solute can be separated from a solution by shaking the solution with a solvent in which the solute is more soluble and added solvent does not mix with the solution. The technique of solvent extraction is mostly applied to separate organic compounds from water.
Q. No. 31: State distribution law or partition law?
Distribution law:
This law states that a solute distributes itself between two immiscible liquids in a constant ratio irrespective of the amount of solute added.
Q. No. 32: Define distribution coefficient.
The ratio of the amounts of solute dissolved in two immiscible liquids at equilibrium is called the distribution coefficient. (KD)
KD= Conc. of solute in organic phase / Conc. of solute in the aqueous phase
Q. No. 33: In the solvent extraction technique, why repeated extractions using a small portion of solvent are more efficient than using a single but large volume of solvent?
It has been experimentally observed that repeated or multiple extractions in which solvent is used in small portions are more efficient than using a single but large volume of solvent because more product is extracted with more extractions using a smaller portion of solvent.
Q. No. 34: A solid organic compound is soluble in water as well as in chloroform. During its preparation, it remains in an aqueous layer. Describe a method to obtain it from this layer.
The organic compound can be extracted by solvent extraction technique, as it is mentioned that the compound is soluble in solvents like water and chloroform. The aqueous solution of the compound is mixed with chloroform (CHCl3). The mixture is put into the separating funnel. Here two layers are formed. The water layer is separated and evaporated to get the compound.
Q. No. 35: What is chromatography? Give its types.
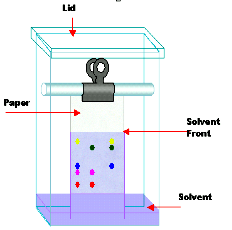
Chromatography: The word chromatography originates from the Greek word “khromatos” meaning colour writing. OR
Chromatography is a method used basically for the separation of the sample of the mixture. OR
It is the process by which different components of a mixture are separated by distributing them stationary phase and mobile phase.
Types: There are two types of chromatography.
- Adsorption chromatography:
- Partition chromatography.
Q. No. 36: What is the principle of chromatography?
The principle of chromatography depends upon two points.
- Components of a mixture are separated due to their relative affinity for stationary and mobile phase.
- Distribution coefficient of components in stationary phase and mobile phase. Distribution coefficient is given as;
KD= Conc. of solute in organic phase / Conc. of solute in the aqueous phase
Q. No. 37: Differentiate between the stationary phase and mobile phase.
Stationary phase: The stationary phase may be solid or liquid supported as a thin film on the surface of an inert solid. It is the phase over which the mobile phase moves. Mobile phase: The solvent or mixture of solvents used for the separation of the components of a mixture in chromatography is called the mobile phase. It is the phase that flows over the surface of the stationary phase. It may be gas or a liquid.
Q. No. 38: Define adsorption and partition chromatography.
Adsorption chromatography:
Chromatography in which the stationary phase is solid is called adsorption chromatography. In this chromatography mobile phase move over a solid surface and just adsorb over it. E.g. thin layer chromatography, column chromatography etc.
Partition chromatography:
Chromatography in which the stationary phase is a liquid is called partition chromatography. In this chromatography mixture (sample) is separated due to the combined interaction of mobile and stationary phases. E.g. Paper chromatography.
Q. No. 39: What is Rf value, give its formula.
Rf stands for retardation factor. And every component in a mixture has its own specific value.
Rf Value: Rf Values is the ratio between the distance travelled by a component from the original spot to the distance travelled by solvent from the original spot.
Rf = Distance travelled by a component from the original spot / Distance travelled by solvent from the original spot
Rf value is always less than 1.0 and it has no unit.
Q. No. 40: Write down applications (uses) of chromatography.
- Chromatography is useful in the separation of organic compounds.
- It is used for the identification of compounds.
- It is useful in qualitative and quantitative analysis.
- It is useful in checking the Purity of the compound. A pure compound gives only one spot.




