These organic conversions are very important chemical reactions for class 12 students from an exam point of view. Printable notes are also available.
Table of Contents.
Important tricky points to learn organic conversions.
The following are very important points to consider when you solve organic conversions. Examples of all points are written in the last in the form of notes and video lecture.
1- Increment of Carbon atom
Number of carbon atoms can be increased by halogenation and Wurtz synthesis.
2- Decrement of Carbon atom
Carbon atoms can be decreased from compound by decarboxylation of carboxylic acid.
3- Increment of the number of bonds
Number of C-C bonds can be increased by either Halogenation + dehydrogenation or by Kolbe’s electrolysis.
4- Decrement of the number of bonds
Number of C-C bonds can be increased by dehydrogenation.
5- Increment of C + formation of COOH
If you have to increase the number of C with the formation of carboxylic acid, then two methods may be used.
i. using Grignard’s reagent reaction with Carbon dioxide and
ii. prepare nitrile (Cyanide) and do its hydrolysis.
6- Oxidation of alkane
By doing oxidation following reactions occur.
(Alkane → alcohol → aldehyde/ketone → carboxylic acid)
7- Reduction of carboxylic acid
By doing reduction following reactions occur. which are contrary to oxidation reactions.
(Carboxylic acid → Aldehyde→ alcohol→ alkane)
To understand the above-mentioned points following examples should be considered.
In a one video lecture you can understand all examples on YouTube. Or pdf notes are also available, you can download from the link below.
Examples:
- 00:12:04 1. Convert Ethane into Ethene
- 00:15:11 2. Convert Ethene into Ethyne
- 00:18:15 3. Covert Ethyne into Ethane
- 00:19:59 4. Convert Methane into Propanoic Acid
- 00:29:43 5. Convert Ethane into Quaternary ethyl ammonium bromide
- 00:38:34 6. Convert Ethene into Butanol
- 00:45:55 7. Convert Propylchloride into Propene
- 00:49:18 8. Convert Acetic acid into Propanoic acid
- 00:54:27 9. Convert Methanol into Ethanol
- 00:57:49 10. Convert Ethanol into methanol
- 01:04:04 11. Convert Ethanol into isopropyl alcohol
- 01:07:34 12. Convert Formaldehyde into Ethyl alcohol
- 01:09:37 13. Convert Acetone into Ethyl Alcohol
- 01:15:52 14. Convert Acetone into t-Butyl Alcohol
- 01:18:51 15. Convert Propanal into 1-Propanol
- 01:20:17 16. Convert Propanone into 2-Propanol
- 01:21:59 17. Convert Methanal into Ethanal
- 01:23:18 18. Convert Ethene into Ethanal
- 01:25:32 19. Convert Ethanal into Propanone
- 01:26:56 20. Convert Ethanal into 2-Propanol
- 01:29:00 21. Convert Methanol into Ethanal
- 01:30:12 22. Convert Ethyne into Ethanal
- 01:33:39 23. Convert Ethanal into Ethanol
- 01:34:19 24. Convert Ethanol into 2-Butanone
- 01:37:37 25. Convert Ethanol into Ethanoic Acid
- 01:38:56 26. Convert Acetic Acid into Acetamide
- 01:39:48 27. Convert Acetic Acid into Acetone
Notes of Organic Conversions.
1- Notes (Handouts) for All Important conversions from Class 12
2- Advanced Notes.
Advanced printable notes cover all the conversions and all organic chemistry reactions on just 10 pages. If you have a passion for learning and preparing for organic chemistry quickly in a short time, then these notes are for you. These notes are premium but 80% off for you for a few days. Now it costs only Rs.100.

Procedure: Transfer your amount to +92-309-9164667 (Jazz Cash) and send a screenshot to WhatsApp at the same number. Notes will be sent to you on the same day.
(Account title: Umair Ali Khan)
For any help, contact me at the same WhatsApp number mentioned above.
For International Students
IBAN: PK38UNIL0109000272372128
Price: $1 only
Important Highlights.
Advanced Notes cover the following conversion reactions and chain reactions.
- Conversion of alkane to all other compounds.
- Conversion of alkene to all other compounds.
- Conversion of alkyne to all other compounds.
- Conversion of alkyl halide to all other compounds.
- Conversion of alcohol to all other compounds.
- Conversion of aldehyde to all other compounds.
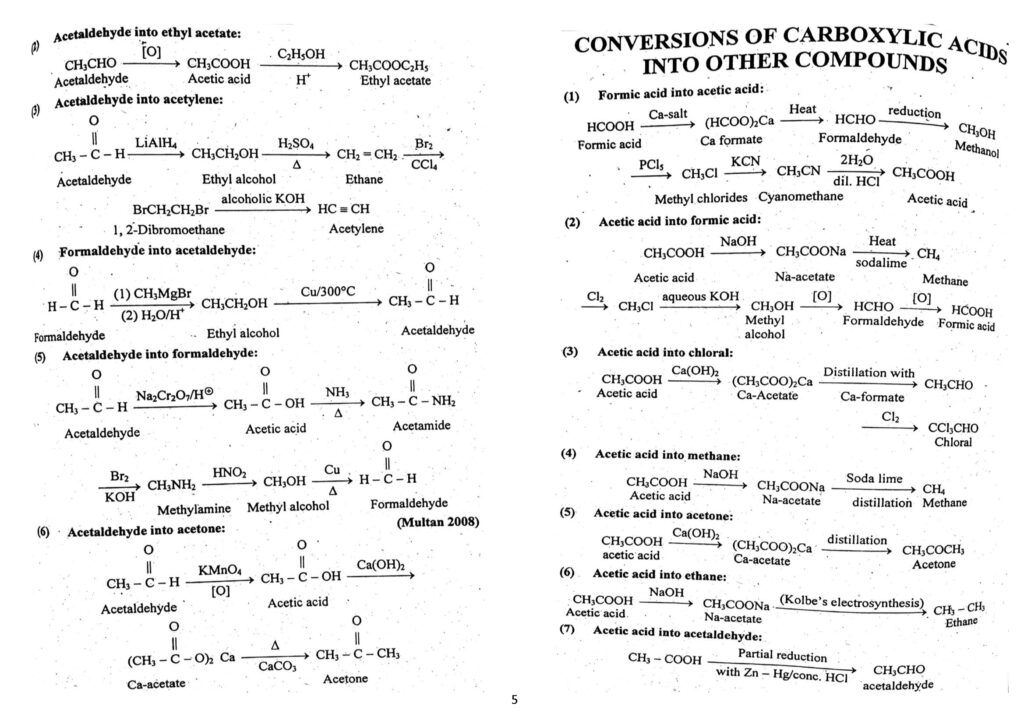
- Conversion of carboxylic acid to all other compounds.
- Conversion of derivatives of benzene to all other compounds.
- Conversion chains from Carbon and Hydrogen.
- Ascending and Descending chains of C chains.
A Screen Shots for reference.






Great sir
Thanks