Umair Khan Academy provides notes, quizzes, video lectures and guidance for its Students. Today Class 12 Chemistry chapter 1 is under study. To read other notes visit Umair Khan Academy.
Extensive and Short questions in the chapter ‘Periodic Classification of Elements and Periodicity’ are given below.
INTRODUCTION AND HISTORICAL BACKGROUND
Q1. Give some historical perspective of the periodic table of elements.
For the systematic study of elements, we need to classify the elements in the form of a periodic table. The periodic table provides a basic framework to study the periodic behaviour of physical and chemical properties of elements as well as their compounds.
HISTORICAL BACKGROUND:
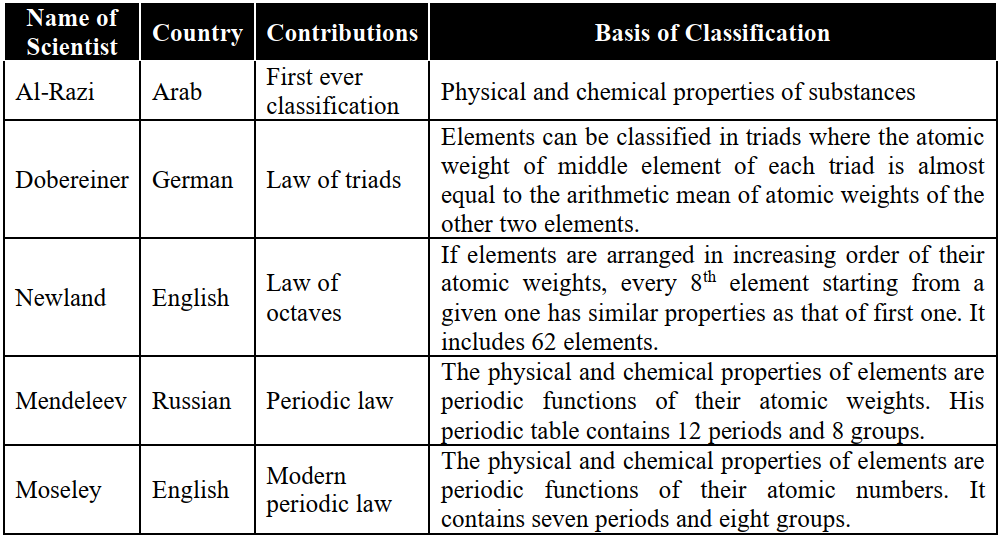
Q2. Write some Advantages and improvements of Mendeleev’s Periodic Table.
Advantages:
- Mendeleev left many vacant spaces in his periodic table for unknown elements and predicted their properties e.g. germanium.
- Mendeleev’s Periodic Table helped to correct the doubtful atomic weights of elements e.g. beryllium.
Improvements:
- Arrangement of elements in ascending order of atomic numbers because Mosely found that atomic number is a more fundamental property than atomic mass.
- Addition of group VIII to place the newly discovered noble gases properly.
- Introduction of two types of vertical groups A and B to eradicate the misplacement of Zn, Cd, Hg and Be, Mg, Ca, Sr, and Ba in the same vertical group and so many others in the same manner.
Q3. Write a comprehensive note on the properties of the Modern Periodic Table.
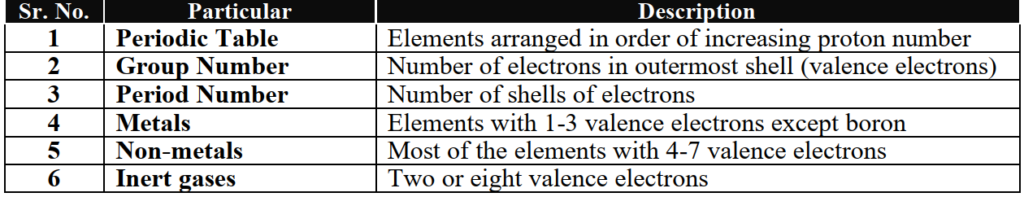
1-Groups and Periods
- Essential Features of Groups
- The vertical columns of elements in the periodic table are called groups. Each group represents number of valence electrons.
- All the elements in a group have similar properties and similar electronic configuration of valence shell.
- There are eight groups that are shown by Roman numerals I to VIII.
- Each group has two sub-groups A and B. ‘A’ contains normal or typical elements while ‘B’ contains transition elements.
- Essential Features of Periods
- The horizontal rows of elements in the periodic table are called periods.
- All elements in a period have a same number of shells according to the period number.
- There are seven periods in the periodic table that are known by Arab numerals 1 to 7.
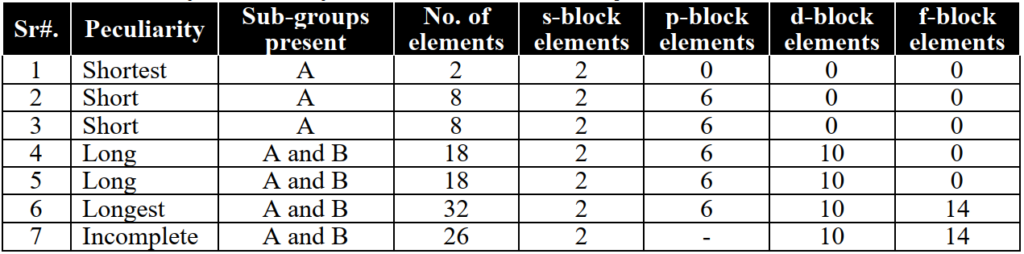
2- Families
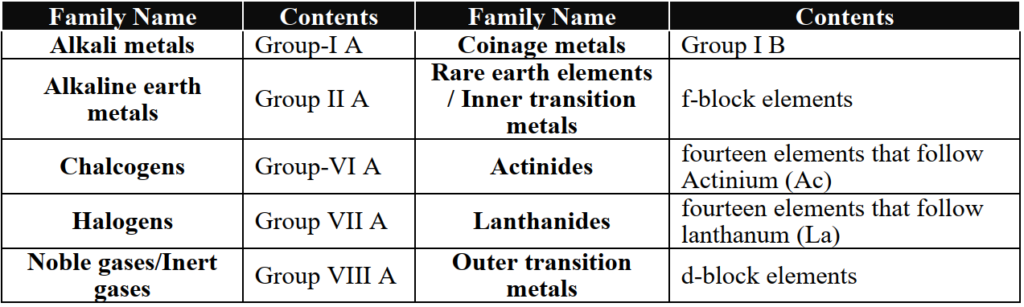
3- Blocks

4- Classification of elements on the basis of metallic characters
- Metals
- Non –metals
- Metalloids
Q4. What is Newland’s law of octaves?
Newland classified sixty-two (62) elements, known at that time, in increasing order of their atomic masses. He noticed that every eight elements had some properties in common with the first one. The principle on which this classification is based is called the Law of Octaves.
Q5. How does the classification of elements in different blocks help us understand their chemistry?
Blocks in the periodic table:
Elements of the periodic table are classified into four blocks on the basis of the last electron present in the specific orbital.
- s-block elements: I-A and II-A group elements and Helium are called s-block because their valence electrons are available in the s-orbital.
- p-block elements: III-A to VIII-A group elements (except He) are called p-block elements because their valence electrons are present in the p-orbital.
- d-block elements: I-B to VIII-B group elements (transition elements) are called d-block elements because their valence electrons are present in the d-orbital.
- f-block elements: Lanthanides and actinides are called f-block elements because their valence electrons are present in the f-orbital.
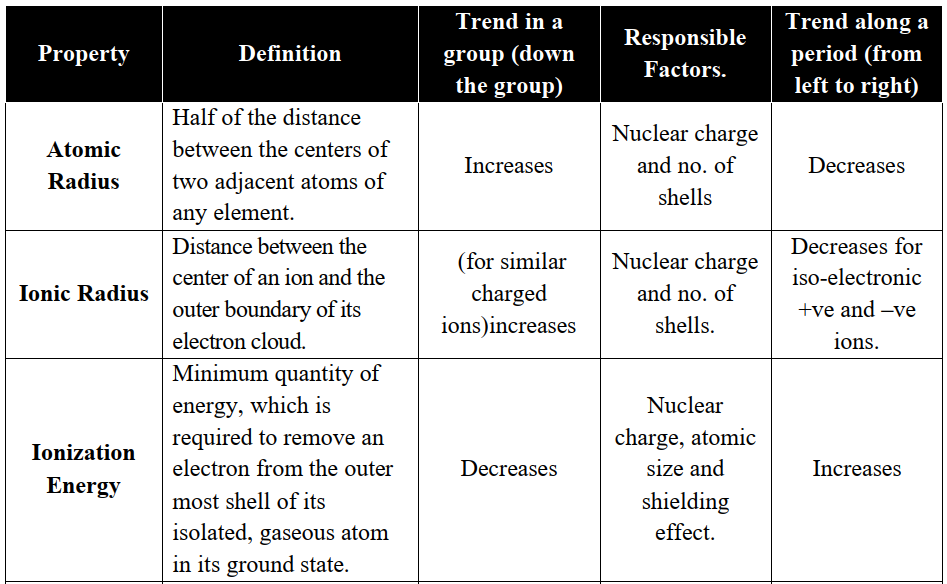
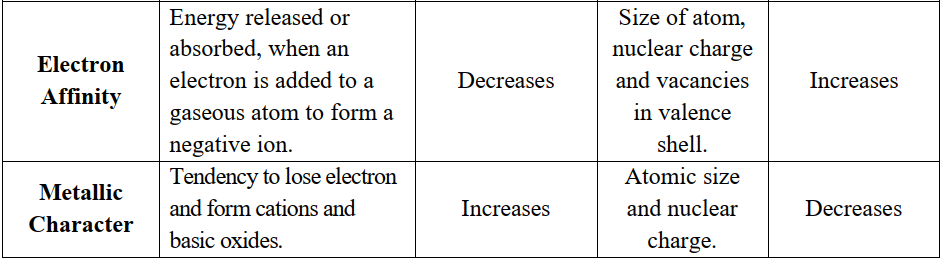
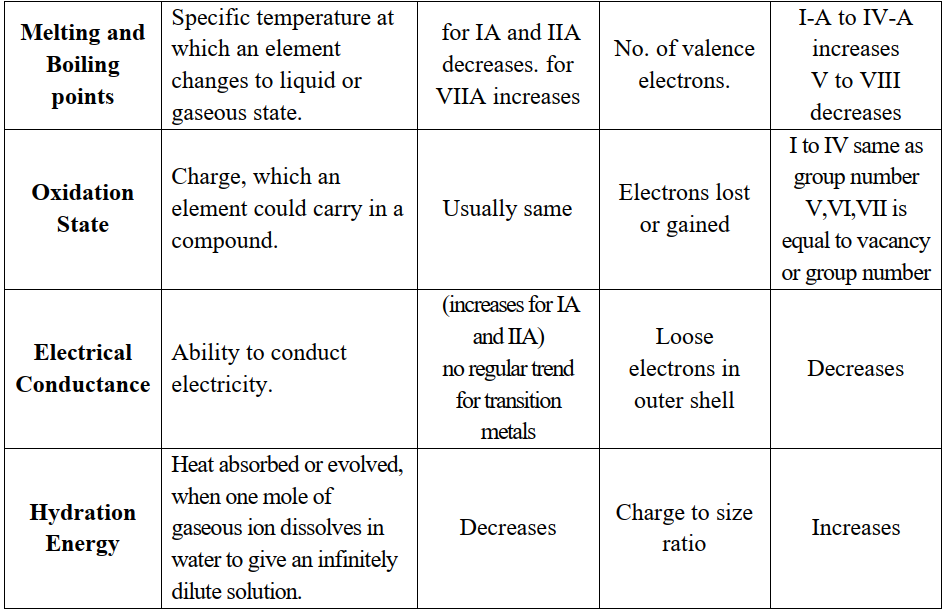
PERIODIC TRENDS IN THE PHYSICAL PROPERTIES
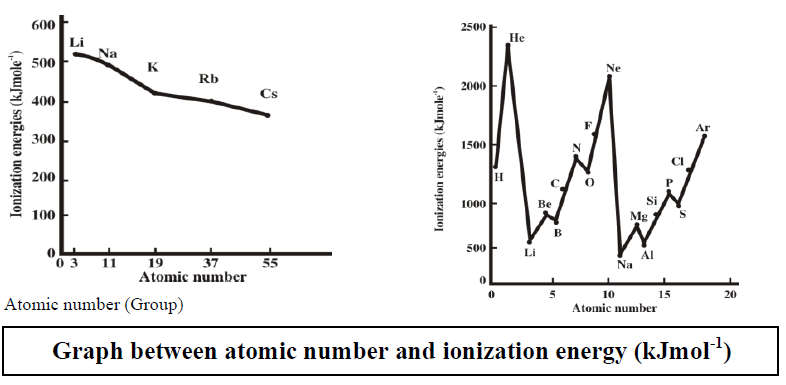
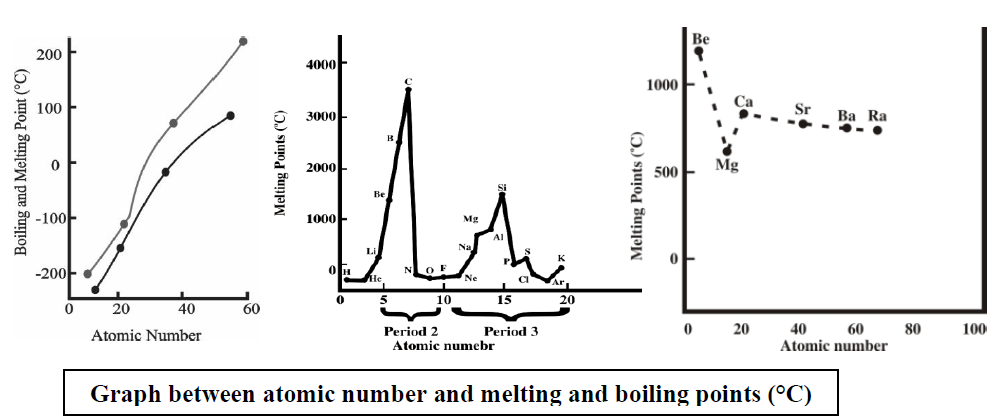
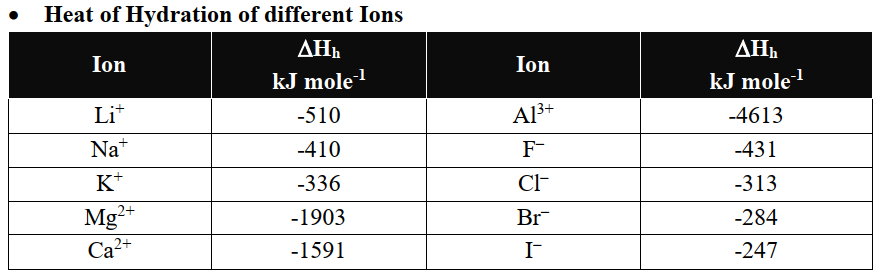
Q6. Write important periodic trends in the physical properties of elements.
The classification in the periodic table is based on the similarities in the properties of elements. But physical and chemical properties of elements vary steadily in a
- Group (due to an increase in the number of shells) or
- Period (due to gradual increase in the proton number in the nucleus and electrons in the valence shell).
VIDEO LECTURES ON PHYSICAL PROPERTIES (Class 12 Chemistry)
Q7. How Lanthanide contraction controls the atomic size of elements of the 6th and 7th periods.(Exam Question in Class 12 Chemistry)
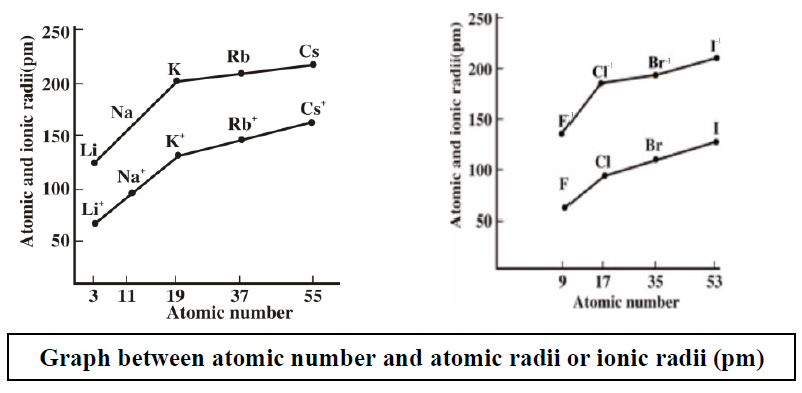
The gradual decrease in the atomic size of the elements in the lanthanide series is significant and is called lanthanide contraction. The same decrease is observed in the actinide series. This is due to the poor shielding effect of the f sub-shell which is being gradually filled along the series. The number of protons is increasing but the number of valence electrons remains the same as electrons are added in f sub-shell of inner shells. Due to the poor shielding of electrons in the f sub-shell, the increasing number of protons pulls the valence electrons strongly. Hence, responsible for the decrease in size.
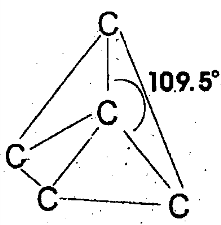
Q8. Why diamond is a non-conductor and graphite fairly a good conductor?
In diamond, the unit cell is tetrahedral. Each carbon atom is sp3 hybridized and forms four sigma bonds with four other carbon atoms and this trend extends throughout the crystal. All four electrons of each carbon atom are tightly bound in these covalent bonds. As a result, no free electrons are available in diamond so it is a non–conductor of heat and electricity.
Graphite is a conductor:-
In Graphite, each carbon atom is sp2 hybridized and bonded to three neighbouring carbon atoms. So, one of its four valence electrons is relatively free to move along the layers. Hence, Graphite is a conductor parallel to the layers and a non-conductor perpendicular to the layer.
PERIODIC RELATIONSHIPS IN COMPOUNDS (Class 12 Chemistry)
Q9. Write the Periodic relationship of HALIDES in the periodic table of elements.
HALIDES
The binary compounds of halogens with other elements are called halides.
1- Classification of Halides in Class 12 Chemistry
- 1. Ionic
- 2. Covalent
- 3. Polymeric
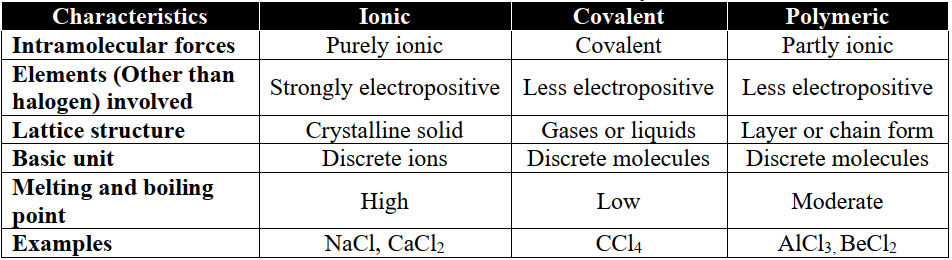
- If an element forms more than one halides, the halide in its lower oxidation state tends to be ionic e.g. PbCl2 is mainly ionic and PbCl4 is fairly covalent due to high polarizing power.
- Order of decreasing ionic character of halides:
Fluoride > Chloride > Bromide > Iodide
2- Bonding Character of Chlorides of the Third Period

Q10. Write the Periodic relationship of HYDRIDES in the periodic table of elements.
The binary compounds of hydrogen with other elements are called hydrides.
Classification of Hydrides in Class 12 Chemistry
- 1. Ionic
- 2. Covalent
- 3. Intermediate
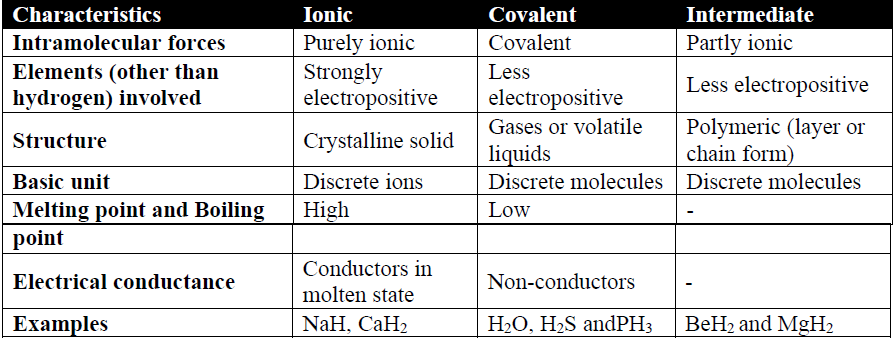
Q11. Write the Periodic relationship of OXIDES in the periodic table of elements.
The binary compounds of oxygen are called oxides.
Classification of Oxides
- On the basis of the nature of chemical bonding
- a) Ionic oxides
- b) Covalent oxide
- c) Neutral oxide
- On the basis of the character of oxide
- (a) Basic oxides (Na2O, K2O, MgO, CaO etc)
- (b) Acidic oxides (CO2, SO3, SiO2, NO2 etc)
- (c) Amphoteric oxides (ZnO, Al2O3, BeO, Ga2O3, In2O3, GeO2, SnO2, PbO, As2O3, Sb2O3, Bi2O3)
POSITION OF HYDROGEN IN THE PERIODIC TABLE
Q12. Justify the position of Hydrogen in the periodic table of elements.
Hydrogen resembles elements of groups IA, IVA and VIIA. Its actual position is still confusing because of its behaviour.
1- Position over Alkali Metals (I-A)
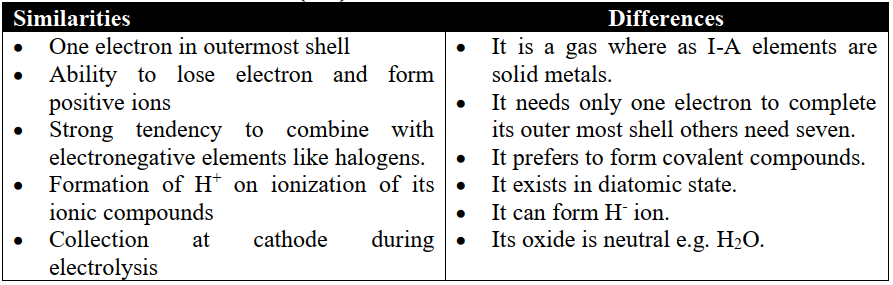
2- Position over Halogens (Group-VII-A)
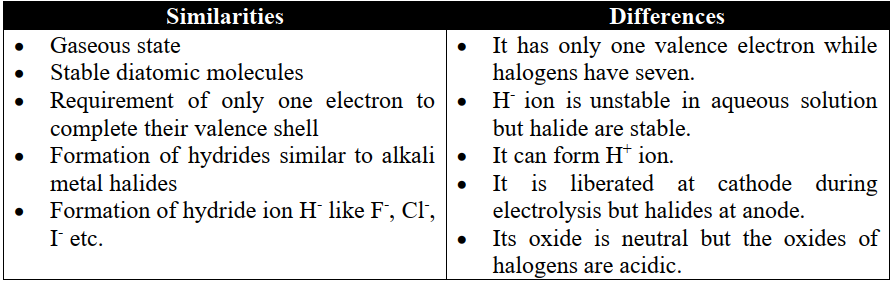
3- Position over Carbon-Silicon Family (Group-IVA)

Q13. Write down similarities of hydrogen with group IV-A elements (Exam Question in Class 12 Chemistry)
- Both have a half-filled valence shell.
- Both combine with other elements to form covalent bonds.
- Hydrogen and top members of IV-A are non-metals.
- Like Carbon, hydrogen also possesses remarkable reducing properties.
SnO2 + C → Sn + CO2
CuO + H2 → Cu + H2O - Hence the position of hydrogen in group IV-A elements is justified.
Q14. Why ZnO is regarded as amphoteric oxide?(Exam Question in Class 12 Chemistry)
Oxides of less electropositive element like ZnO behaves as amphoteric oxide because it
behaves as an acid towards strong bases and as a base towards strong acids.
Example:
ZnO + H2SO4 (Acid) → ZnSO4 + H2O
ZnO + NaOH (Base) + H2O → Na2[Zn(OH)4]
GET IN TOUCH
Visit YouTube Channel
