MCQ- 6
Which of the following is/are true in the cell shown below?
I. Electrons flow through the met from left to right
II. Cu is an anode
III. The spontaneous reaction is
Cu2+ + Zn → Cu + Zn2+
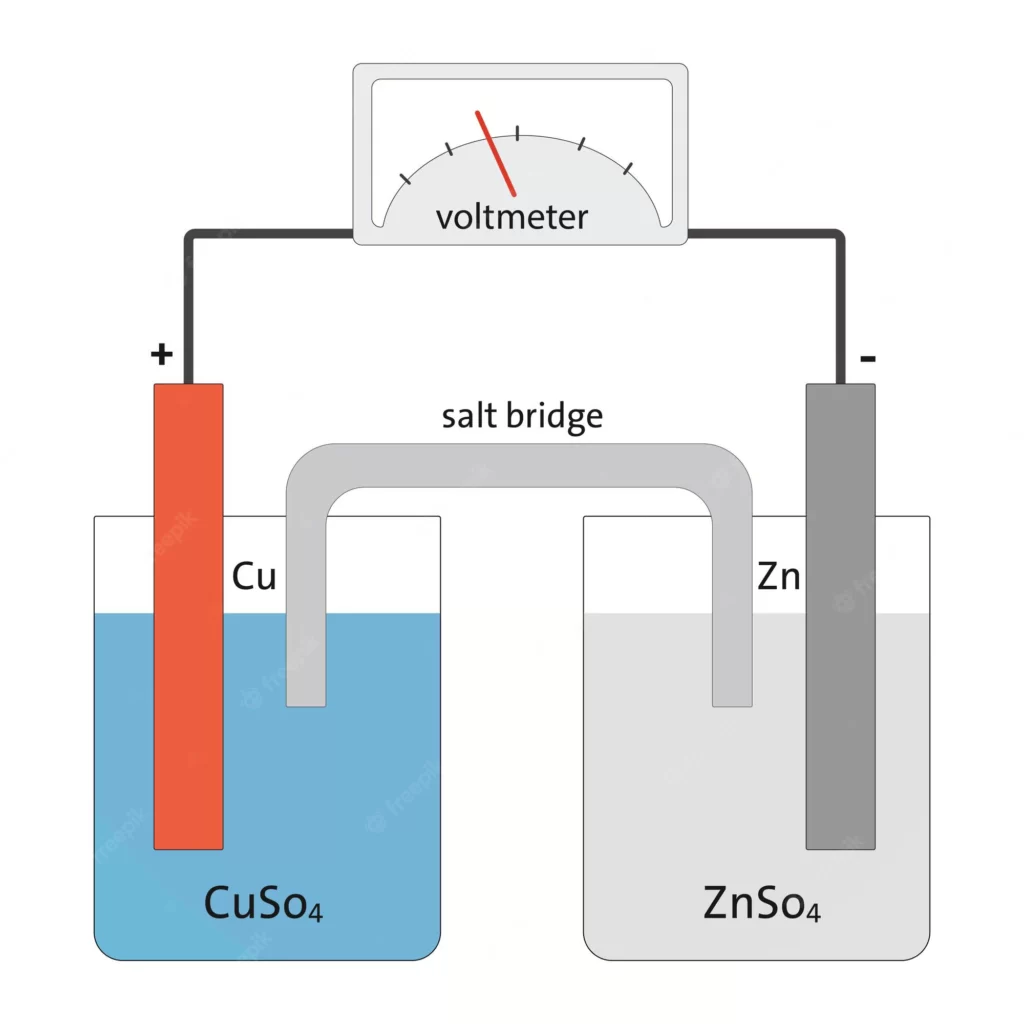
A. I only
B. III only
C. II only
D. I and II
Answer: B
MCQ- 7
The net heat change in a chemical reaction is the same whether it is brought in two or more different ways in one or several steps. It is known as
A. Henry’s law
B. Joule’s principle
C. Hess’s law
D. Law of conservation of energy
Answer: C
MCQ- 8
Solutions containing chlorate (I) ions are used as household bleaches and disinfectants. These solutions decompose on heating as shown;
3ClO– → ClO3– + 2Cl–
Which of the following ion will have the highest oxidation state
A. ClO–
B. ClO2–
C. ClO3–
D. Cl–
Answer: C
MCQ- 9
Which of the following is a list of metals in order from strongest to weakest reduction agents?
A. Au > Ni > Ag
B. Ni > Au > Ag
C. Ni > Ag > Au
D. Ag > Ni > Au
Answer: C
MCQ- 10
Which of the following will be collected at the cathode during electrolysis of aq. CuSO4 solution?
A. H2
B. O2
C. SO3
D. Cu
Answer: D




test is good but some mcqs are out of syllabus
Thank you for your feedback, MCQs will be updated as soon as possible.