31. Calculate the mass in grams of 2.74 moles of KMnO4? The formula mass of KMnO4 is 158 g/mol.
| Moles of KMnO4(n) | = 2.74 mole |
| Molar mass (M) | = 39+55+16×4 = 158 g/mole |
| 1 mole of KMnO | = 158 g |
| Moles of KMnO4 | = Mass / Formula maass |
| Mass | = Moles x formula mass |
| = 2.74 x 158 | |
| =432.92 g |
32. What is a mass spectrum?
Mass spectrum: In modern spectrographs, each ion strikes a detector, and the ionic current is amplified and fed to the recorder. The recorder makes a graph showing the relative abundance of isotopes plotted against the mass number.
33. Give the main points for the determination of molecular formula?
There are the following steps involved.
- Determination of percentage composition by combustion analysis.
- Calculate the number of moles of each element.
- Find out the simplest atomic ratio of all elements in a compound.
- Determination of empirical formula from simplest ratio.
- Determination for a molecular formula using the following expressions
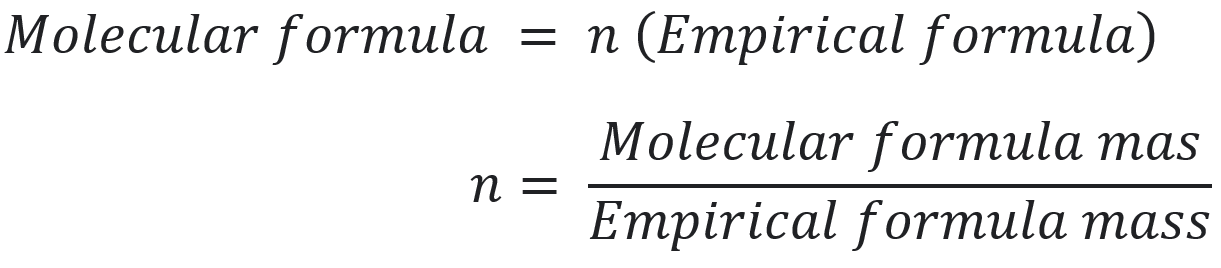
34. How the isotopes of an element are separated by a mass spectrometer?
Isotopes of an element are separated on the basis of their m/e ratio.
35. Why percentage of oxygen cannot be determined directly in combustion analysis?
As oxygen is necessary for combustion analysis so it is used in excess. Combustion of organic compound converts “C” into CO2 and “H” into H₂O as:
C + O₂ → CO₂
H2+1/2 O2 → H2O
The amount of oxygen is determined by the method of difference.
36. Write down four steps for the determination of the empirical formula?
\An empirical formula can be determined by using following steps:
- Determination of percentage composition.
- Finding the number of gram atoms of each element.
- Determine the atomic ratio of each element.
- If the atomic ratio is a simple whole number it gives the empirical formula, otherwise multiply with a suitable digit to get the whole number atomic ratio.
37. Calculate the number of molecules in 9 g of ice?
| Given mass of H₂O(m) | = 9 g |
| Molar mass of H2O(M) | = 18 g/mole |
| No. of molecules of H2O in 9g of ice(N) | =? |
| Formula: N | = m/M × NA =9/18 × 6.022 ×1023 =3.01×1023 |
38. Why atoms cannot be observed by an ordinary optical microscope?
An ordinary optical microscope can measure the size of an object up to or above 500nm. But the size of an atom is smaller. So, a clear and accurate image of an object that is smaller than the wavelength of visible light cannot be obtained using an ordinary microscope.
39. How can the efficiency of a chemical reaction be expressed?
The efficiency of a reaction is expressed by comparing the actual and theoretical yields in the form of percentage yield.![]()
40. Define stoichiometry and Molar volume?
Stoichiometry:
The branch of chemistry deals with the study of the quantitative and qualitative relationships between reactants and products in a balanced chemical equation.
Molar Volume:
A volume occupied by one mole of an ideal gas is called molar volume. It is 22.414dm3 for an ideal gas.



