If you want to improve yourself then Quiz solving is the best way. Fortunately, Umair Khan Academy is providing you with such a facility. So go ahead and achieve your goals with great ease.
Start your quiz by clicking the START button below
Quick Revision chapter 1 class 12
Historical Background
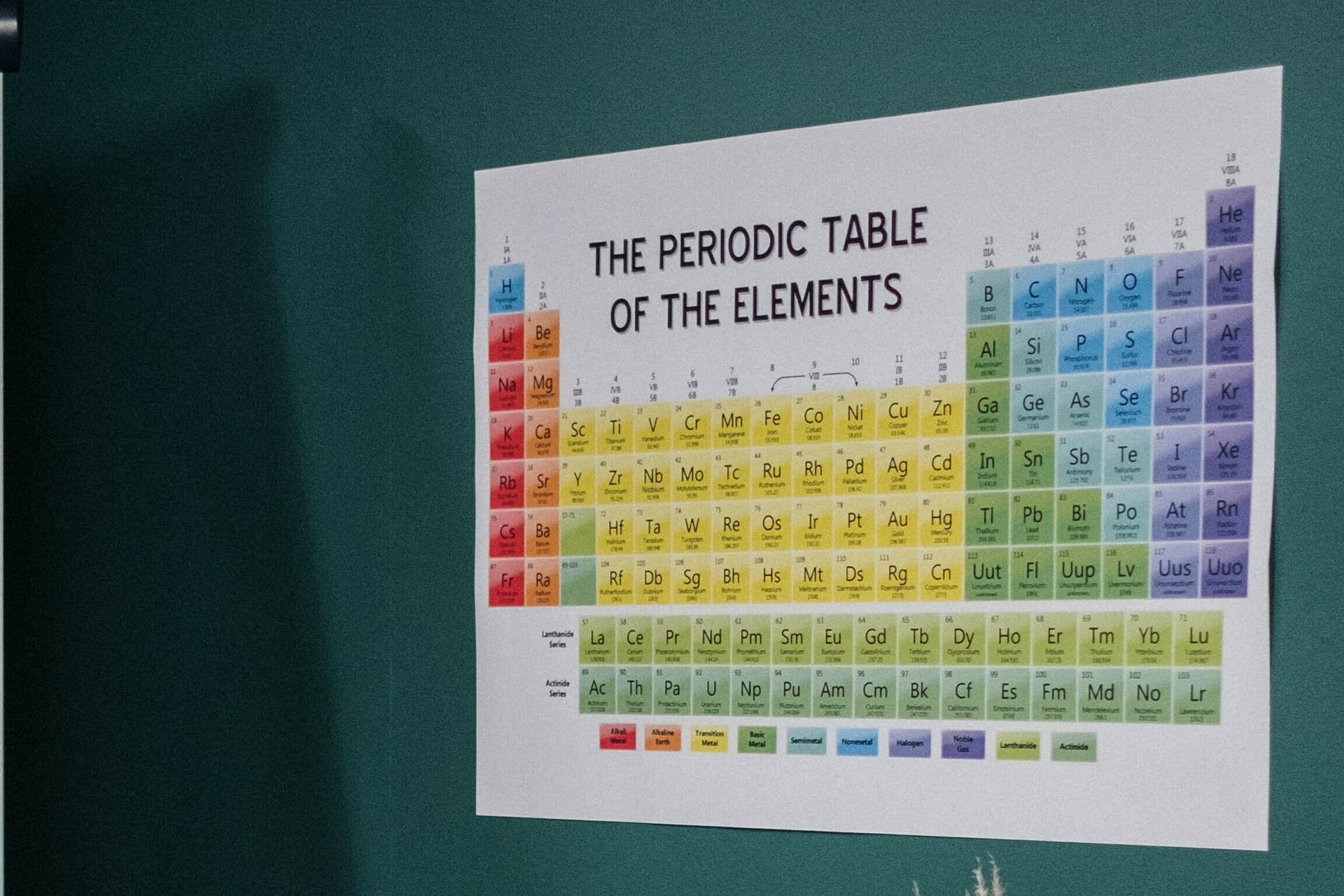
(1) In the history of chemical sciences, significant movements are there in the development of the periodic table.
(2) Periodic table gives us information about the periodic properties.
(3) The scientists who have contributed to the periodic table are Al-Razi, Doberiener, Newland, Meyer, Mendeleev and Moseley.
(4) Mendeleev’s table helped to predict the position of new elements and corrected the atomic masses.
(5) Mendeleev’s table could not fix the portion of hydrogen, lanthanides actinides and isotopes.
(6) Moseley said that the physical and chemical properties of elements are
periodic functions of their atomic numbers.
(7) Moseley corrected the position of some elements, gave positions to rare earths and adjusted the isotopes, rare earths, noble gases and coinage metals in the periodic table.
MODERN PERIODIC TABLE
(8) In the modern periodic table, there are eighteen groups. Sub-group A are of normal or representative elements, while sub-group B are of transition elements.
(9) Period 1 has two elements, 2 and 3 have eight, 4 and 5 have eighteen, 6 has thirty-two while period 7 has twenty-four elements in the periodic table.
(10) Periods 4, 5, 6 and 7 have transition elements along with representative
elements.
(11) Period 7 is incomplete.
SPECIFIC NATURE OF GROUPS AND PERIODS
(12) Elements having atomic number more than 92 are called transuranic elements and they have atomic numbers from 93 to 110.
(13) Lanthanides and actinides are of 45 and 5f series respectively and are also called rare earths. They are placed outside the periodic table and belong to the 6th and 7th periods respectively.
(14) Elements of groups 1-A and II-A are called alkali and alkaline earths. They are also called s-block elements.
(15) Elements of VI-A and VII-A are called chalcogens and halogens
respectively and both are p-block elements.
(16) VIII-group of representative elements consists of noble gases or inert gases.
(17) Elements of groups I-A and II-A are s-block, from III-A to VII-A and VIII-A are p-block elements. Collectively, they are called representative elements.
METALLIC AND NON-METALLIC NATURE
(18) Elements of groups 1-A, II-A, along with d and f-block elements are metals.
(19) The upper elements of IV-A, V-A, VI-A, VII-A are non-metals.
(20) The metallic character increases from top to bottom and decreases from left
to the right in the periodic table.
(21) Metals lose electrons easily and are good conductors of heat and electricity. They are malleable, ductile and give basic oxides.
(22) Non-metals accept the electrons easily, are not good conductors of heat and electricity and give acidic oxides.
(23) The properties of metalloids are intermediate between metals and non-metals and give amphoteric oxides.
POSITION OF HYDROGEN
(24) Hydrogen can be placed in groups I-A, IV-A and VII-A. It resembles and differs from these elements in many of its properties.
ATOMIC AND IONIC RADII
(25) The atomic sizes are assessed in terms of atomic radii, ionic radii, covalent radii or van der Waal’s radii. They are expressed in nm, Aº or pm.
(26) There are no sharp boundaries of atomic orbitals.
(27) Atomic radii, ionic radii and covalent radii decrease from left to right in periods and increase from top to the bottom in a group.
(28) The factors which affect the atomic sizes are nuclear charge, number of
shells and shielding effect of inner electrons.
(29) The sizes of positively charged ions are smaller than neutral atoms, and negatively charged ions are larger in sizes than neutral atoms.
(30) Greater the amount of positive charge, the smaller the size and the greater the amount of negative charge, the bigger the sizes.
IONIZATION ENERGY
(31) Ionization energy (IE) is the quantitative measure of the force of attraction between the nucleus and the outermost electron. It is measured in kJ/mol. Its value is influenced by nuclear charge, number of shells, shielding effect and nature of orbitals.
(32) Ionization energy values increase from left to right and decrease from top to the bottom in a group. The elements of groups III-A and VI-A show abnormally low values of I.E. as compared to the values of II-A and V-A respectively.
(33) Second ionization energy values of elements are greater than the first I.E. values. From the sharp changes of ionization energy values, we can guess the valencies of the elements. Electron affinities and ionization energies are expressed in kJ /mol.
(34) All the ionization energies are positive.
ELECTRON AFFINITY
(35) First electron affinities of most of the elements are negative, while second and third electron affinities are always positive.
(36) Electron affinities increase from left to right and decrease from top to the bottom. Elements of groups II-A, V-A and VIII show the abnormal trend in their values.
(37) The electron affinities of second-period elements (Lis – Neo) are less than those of the third period due to their extremely small sizes.
MELTING POINTS AND BOILING POINTS
(38) Melting and boiling points decrease down the group for the first four groups of the representative elements. These values increase from top to the bottom for the last four groups of representative elements.
(39) In a period from left to right, the melting and boiling points increase up to group IV-A and then they decrease.
(40) In the case of transition metal series, the melting and boiling points increase up to the middle of the series and then they decrease.
OXIDATION STATES
(41) Oxidation number of an element is closely related to the electronic distribution and it depends upon the group number. Anyhow, for group V-A, VI-A and VII-A, the oxidation numbers increase from top to bottom due to unpairing of electrons.
(42) The maximum oxidation state for sulphur is +6 and for halogens +7. Transition elements also show the variable oxidation states.
ELECTRICAL CONDUCTIVITY
(43) Electrical conductivity depends upon the metallic and non-metallic nature. It changes with the number of free electrons. It decreases from top to the bottom for the elements of group I-A and II-A with few exceptions. Coinage metals (Cu, Ag. Au) are excellent conductors.
(44) Diamond is a bad conductor of electricity but graphite is a good conductor and current is carried parallel to the layers. This is the anisotropic behaviour of graphite.
HYDRATION ENERGY
(45) Hydration energy of the ion depends upon the charge density or charge to size ratio. Greater the charge density of the positive or negative ion, the greater the heat of hydration.
(46) Values of hydration energies increase from left to right in a period of isoelectronic species.
HALIDES
(47) Halides are of three types i.e. ionic, polymeric and covalent.
(48) The ionic character of the halides decreases from left to right and increase from top to the bottom. Ionic halides are hard crystalline substances, while covalent halides are soft.
(49) Greater the oxidation state of the central atom, the greater the covalent character of halides. Moreover, the chlorides of a metal are more ionic in nature as compared to bromide and iodides of the same metal.
HYDRIDES
(50) There are six types of hydrides, but the most important are ionic, covalent, metallic and complex.
(51) Ionic hydrides are given by groups I-A and II-A except Be and Mg, which give polymeric hydrides.
(52) Hydrides of groups III-A, IV-A, V-A, VI-A, VII-A are covalent in nature and they become more and more acidic from left to right.
(53) The melting and boiling points of covalent hydrides depend upon the hydrogen bonding present in them or random dispersion forces.
OXIDES
(54) There are three types of oxides i.e. basic, acidic and amphoteric.
(55) Metals give basic oxides, non-metals give acidic and metalloids give amphoteric oxides.
(56) The acidic character of oxides increases from left to right and decreases from top to the bottom.
(57) Greater the oxidation number of the metals and non-metals making the oxide, greater the acidic strength of oxide. SO3 is more acidic than SO2, N₂O5 is more acidic than N₂O4, and Mn₂O7 is more acidic than MnO2.



